Abstract
We systematically analyzed and attempted to discuss the possibility that deficiencies of zinc or selenium were associated with the incidence and severity of COVID-19. We searched for published and unpublished articles in PubMed, Embase, Web of Science and Cochrane up to 9 February 2023. And we selected healthy individuals, mild/severe, and even deceased COVID-19 patients to analyze their serum data. Data related to 2319 patients from 20 studies were analyzed. In the mild/severe group, zinc deficiency was associated with the degree of severe disease (SMD = 0.50, 95% CI 0.32–0.68, I2 = 50.5%) and we got an Egger’s test of p = 0.784; but selenium deficiency was not associated with the degree of severe disease (SMD = − 0.03, 95% CI − 0.98–0.93, I2 = 96.7%). In the surviving/death group, zinc deficiency was not associated with mortality of COVID-19 (SMD = 1.66, 95%CI − 1.42–4.47), nor was selenium (SMD = − 0.16, 95%CI − 1.33–1.01). In the risk group, zinc deficiency was positively associated with the prevalence of COVID-19 (SMD = 1.21, 95% CI 0.96–1.46, I2 = 54.3%) and selenium deficiency was also positively associated with the prevalence of it (SMD = 1.16, 95% CI 0.71–1.61, I2 = 58.3%). Currently, serum zinc and selenium deficiencies increase the incidence of COVID-19 and zinc deficiency exacerbates the disease; however, neither zinc nor selenium was associated with mortality in patients with COVID-19. Nevertheless, our conclusions may change when new clinical studies are published.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10534-023-00501-0.
Keywords: Zinc, Selenium, Coronavirus disease 2019, Meta-analysis
Introduction
Since the first known case was reported in December 2019, the World Health Organization (WHO) has declared the coronavirus disease 2019 (COVID-19) to be a nationwide pandemic. The severity of COVID-19 is connected to the body's immunity and the viral subtype of infection. Medical researchers exploring COVID-19 therapy have shown that trace elements play a significant role in maintaining immunity and treating viral infections. Among them, zinc and selenium may have favorable immune-modulatory properties (Jayawardena et al. 2020).
Zinc and selenium, which have antiviral and immunomodulatory capabilities, are components of antioxidant enzymes that can suppress virus reproduction in host cells and have a function in sustaining immunity and virus elimination (Pour et al. 2021). Low levels of zinc cause dysfunction in all immune cells, and altered zinc state have a higher risk for infection with COVID-19 (Wessels et al. 2020). The studies have also suggested a correlation between COVID-19 and selenium deficiency (Wessels et al. 2022).Therefore, we should pay attention to any variations in the trace element levels in the serum. In vitro, zinc can stop Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) from replicating and help with the immune response. Changes in hematological and immunological processes brought on by even a slight zinc deficit can result in pro-inflammatory phenotypes and redox metabolic diseases (Wessels et al. 2022). And a risk factor for persistent hypozincemia was identified as a severe case of COVID-19 (Yasui et al. 2020). Meanwhile, we learned from the clinical data that the COVID-19 patients showed a pronounced deficit in total serum selenium concentrations (Moghaddam et al. 2020). Therefore, we indicate these trace elements have such a function in the infection and severity of COVID-19.
This paper presented an extended series of discussion studies based on serum levels aimed at systematically assessing the relationship between micronutrients and the incidence of COVID-19 and its associated severity. We attempted to analyze the effect of microelements deficiency on the incidence of COVID-19 and its severity, to provide some basis for a subsequent randomized controlled trial.
Materials and methods
Search strategy
From database establishment to 9 February 2023, two researchers retrieved relevant peer-reviewed literature on zinc and selenium status in COVID-19 from PubMed, Embase, Web of Science and Cochrane databases. The search keywords included: (1) zinc; (2) selenium; (3) trace element; (4) corona virus OR COVID-19 OR Coronavirus Disease 2019 OR SARS-CoV2 OR SARS-CoV-2 OR 2019-nCoV. Searches were not restricted by language, study design, or country of origin. This study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). We also manually examined the references of the included publications for other relevant investigations. All studies were independently searched and evaluated by the two researchers. Disagreements were settled via consensus.
Inclusion and exclusion criteria
Studies were eligible for inclusion if the following criteria were all met: (1) evaluation of serum zinc or selenium or both; (2) participants were confirmed as COVID-19 patients and healthy human volunteers; (3) detailed prevalence or death information; (4) the number of patients ≥ 30; And exclusion criteria were: (1) case reports, reviews, conferences, meeting abstracts, meta-analyses, letters, comments; (2) data could not be extracted; Two investigators independently completed this process without language restriction, and any discrepancies were resolved by the third investigator.
Data extraction
We extracted the following data from the included studies: the first author, publish year, study design, demographic information of participants (age and gender), number of participants, type of trace element, serum levels in COVID-19 patients and healthy human volunteers, and serum levels in COVID-19 patients who have recovered and COVID-19 patients who have died, serum levels in mild and severe COVID-19 patients, and method for determination of trace elements. Data were tabulated independently by two researchers, and disagreements were resolved by negotiation.
Statistical analysis
All analyses were performed using Stata 16.0 (Stata Corporation, College Station, Texas, USA). In this analysis, zinc and selenium were calculated separately. And random effects models were used to calculate standardized mean differences (SMD) and the corresponding 95% confidence intervals (CI), which due to different data measurement methods or scales. If the original study provided median and interquartile intervals for serum micronutrient levels, we converted them to mean and standard deviation using validated formulas (Luo et al. 2018; Wan et al. 2014). Quality and risk of bias could be assessed through Newcastle–Ottawa Scale (NOS), funnel plots and Egger’s regression asymmetry test will be displayed if necessary.
Heterogeneity between pooled studies will be assessed using Cochrane Q and I2 statistics, with the values of I2 separated by 50%, representing low and high heterogeneity (McGuinness and Higgins 2020). Meta-regression analysis or sensitivity analysis will conduct to evaluate the probable group differences if existing considerable heterogeneity. Covariates will be based on severity of COVID-19 patients and referred to (1) number of patients included; (2) race; (3) study design.
Results
Literature search
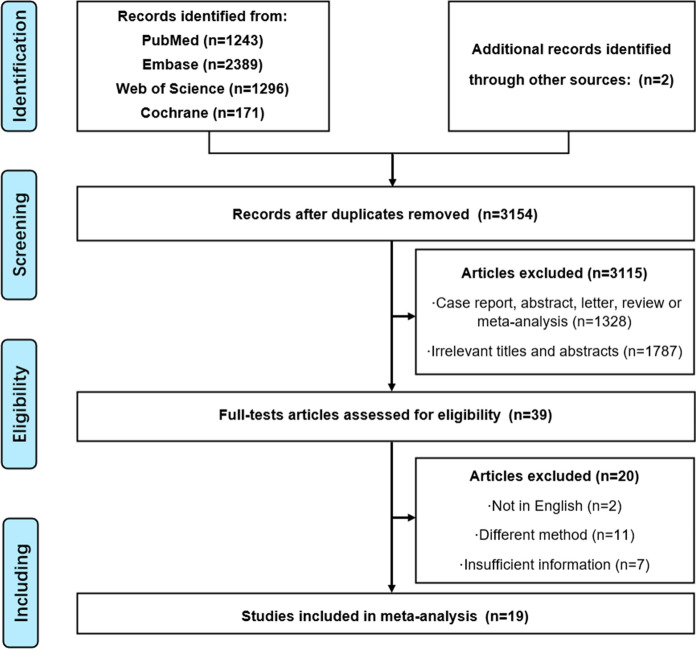
Figure 1 showed the flow chart of literature retrieval. The electronic database search initially identified 5101 articles. Of these, 3115 studies were excluded as irrelevant after duplicating. The remaining 39 articles were carefully reviewed by full text to determine if they met the inclusion criteria and 19 articles were identified. Therefore, 2043 participants were evaluated for zinc and 1184 participants for selenium (Pour et al. 2021; Moghaddam et al. 2020; Shakeri et al. 2022; Ivanova et al. 2022; Zeng et al. 2021; Xu et al. 2022; Hosseini et al. 2021; Golabi et al. 2021; Elham et al. 2021; Voelkle et al. 2022; Al-Saleh et al. 2022; Skalny et al. 2021; Muhammad et al. 2021; Bego et al. 2022; Kocak et al. 2022; Almasaud et al. 2023; Younesian et al. 2022; Jahromi et al. 2021; Majeed et al. 2021; Laing et al. 2021). Details of the selected studies of zinc and selenium were summarized in Tables 1 and 2, respectively.
Fig. 1.
Flow diagram depicting the steps of database searching and research selection in accordance with the preferred reporting items for systematic reviews and meta analyses standards
Table 1.
Characteristics and outcomes of zinc in serum
| Author, year | Country | Study design* | Age (SD) | Total patients (% Men) | Micronutrient | Trace elements analysis** | Serum level, n (mean ± SD)*** | ||
|---|---|---|---|---|---|---|---|---|---|
| Health vs patient | Recovered vs dead | Non-severe vs severe | |||||||
| Shakeri (2022), 2021 | Iran | Retro | 53.0 | 293(50.17%) | Zinc | Kit | NA | NA vs 42(94.17 ± 25.95) µg/dL | 214(118.8 ± 34.40) vs 37(98.83 ± 30.49) µg/dL |
| Ivanova (2022) | Italy | Retro | NA | 97(44.33%) | Zinc | AAS | NA | NA | 22(14.9 ± 3.72) vs 75(12 ± 3.71) µmol/L |
| Zeng (2021), 2021 | China | Retro | 63.0 | 306(48.40%) | Zinc | ICP-MS | NA | 291(6.2 ± 0.85) vs 15(6.35 ± 0.97) µg/dL | 202(6.6 ± 1.0) vs 104(6.22 ± 0.84) µg/dL |
| Xu (2022), 2022 | China | Retro | 49.5 | 114(47.00%) | Zinc | Kit | 38(15.25 ± 5.32) vs 114(6.65 ± 7.88) nmol/mL | NA | NA |
| Hosseini (2021), 2021 | Iran | CS | 54.1 | 56(73.21%) | Zinc | AAS | 44(82.10 ± 17.96) vs NA mg/dL | NA | 24(78.72 ± 22.58) vs 32(72.1 ± 18.18) mg/dL |
| Golabi (2021), 2021 | Iran | CS | 41.0 | 53(68.00%) | Zinc | AAS | 54(114 ± 13) vs 54(101 ± 18) µg/dL | NA | NA |
| Elham (2021), 2021 | Iran | CCS | 51.0 | 93(44.10%) | Zinc | AAS | 186(86.66 ± 11.76) vs 93(67.61 ± 15.10) µg/dL | NA | NA |
| Voelkle (2022), 2022 | Switzerland | Pro | 67.0 | 57(60.00%) | Zinc | ICP-MS | NA | NA | 12(11.66 ± 3.10) vs 10(9.60 ± 2.41) µmol/L |
| Saleh (2022), 2022 | Saudi Arabia | Retro | 50.0 | 155(49.68%) | Zinc | ICP-MS | NA | NA | 49(0.986 ± 0.724) vs 22(1.30 ± 1.81) µg/mL |
| Pour et al. (2021), 2021 | Iran | Pro | 56.4 | 226(50.44%) | Zinc | Kit | NA | 170(69.66 ± 1.34) vs 56(62.43 ± 1.81) µg/dL | 114(68.42 ± 1.35) vs 112(67.3 ± 1.79) µg/dL |
| Skalny (2021), 2021 | Russia | CS | NA | 150(NA) | Zinc | ICP-MS | 44(0.96 ± 0.13) vs NA µg/mL | NA | 100(0.92 ± 0.17) vs 50(0.87 ± 0.22) µg/mL |
| Muhammad et al. (2021), 2021 | Nigeria | CS | 43.8 | 50(70.00%) | Zinc | Kit | 21(64.9 ± 6.2) vs 50(58.1 ± 7.0) µg/dL | NA | NA |
| Bego et al. (2022), 2022 | Bosnia and Herzegovina | Pro | NA | 210(59.52%) | Zinc | ICP-MS | NA | NA vs 52(574 ± 218) µg/L | 51(814 ± 164) vs 53 (679 ± 195) µg/L |
| Kocak et al(2022), 2021 | Turkey | Pro | 48.8 | 60(53.33%) | Zinc | ICP-MS | 32(873.44 ± 335.38) vs NA µg/L | NA | 15(600.64 ± 181.40) vs 13(564.73 ± 180.87) µg/L |
| Almasaud et al. (Almasaud et al. 2023), 2023 | Saudi Arabia | Pro | 56.2 | 123(NA) | Zinc | ICP-MS | 48(11.9 ± 1.8) vs 123(8.8 ± 2.3) µmol/L | 55(8.4 ± 1.8) vs 26(7.9 ± 2.6) µmol/L | 42(10.1 ± 2.2) vs 40(8.2 ± 2.3) µmol/L |
*Retro retrospective study; CS cross-sectional study; CCS case–control study; Pro prospective study
**AAS atomic absorption spectrometer; ICP-MS inductively coupled plasma mass spectrometry; TXRF total reflection x-ray fluorescence
***Mild and moderate cases were referred to as non-severe cases, and it represents mild if there are both mild and moderate
Table 2.
Characteristics and outcomes of selenium in serum
| Author, year | Country | Study design* | Age (SD) | Total patients (% Men) | Micronutrient | Trace elements analysis** | Serum level, n (mean ± SD)*** | ||
|---|---|---|---|---|---|---|---|---|---|
| Health vs patient | Recovered vs dead | Non-severe vs severe | |||||||
| Voelkle et al. (2022), 2022 | Switzerland | Pro | 67.0 | 57(60.00%) | Selenium | ICP-MS | NA | NA | 21(0.9 ± 0.3) vs 8(1.0 ± 0.3) µmol/L |
| Saleh (2022), 2022 | Saudi Arabia | Retro | 50.0 | 155(49.68%) | Selenium | ICP-MS | NA | NA | 49(78.36 ± 18.04) vs 22(76.6 ± 23.54) µg/mL |
| Pour et al. (2021), 2021 | Iran | Pro | 56.4 | 226(50.44%) | Selenium | AAS | NA | 170(125.77 ± 2.41) vs 56(129.15 ± 3.91) µg/dL | 114(123.06 ± 2.58) vs 112(130.19 ± 3.19) µg/dL |
| Younesian et al. (2022), 2022 | Iran | CS | 56.0 | 50(62.00%) | Selenium | AAS | 50(91.7 ± 16.7) vs 50(77.8 ± 13.9) μg/L | 37(77. 9 ± 14.3) vs 17(77.2 ± 12. 3) μg/L | NA |
| Jahromi et al. (2021), 2021 | Iran | Pro | NA | 84(56.00%) | Selenium | AAS | NA | NA | 38(47.07 ± 20.82) vs 19(29.86 ± 11.48) µg/dL |
| Majeed et al. (2021), 2021 | India | Retro | 40.5 | 30(80.00%) | Selenium | ICP-MS | 30(79.1 ± 10.9) vs 30(69.3 ± 8.8) µg/dL | NA | NA |
| Du Laing et al. (2021), 2021 | Belgium | CS | NA | 79(69.62%) | Selenium | TXRF | NA vs 79(59.2 ± 20.6) µg/L | NA | 10(63.1 ± 18.3) vs 39(46.7 ± 17.8) µg/L |
| Moghaddam et al. (2020), 2020 | Germany | CS | 77 | 33(58.00%) | Selenium | TXRF | NA | 27(53.3 ± 16.2) vs 6(40.8 ± 8.1) µg/L | NA |
| Skalny et al. (2021), 2021 | Russia | Pro | NA | 150(NA) | Selenium | ICP-MS | 44(102 ± 16) vs NA µg/dL | NA | 100(93 ± 20) vs 50(87 ± 31) µg/dL |
| Muhammad et al. (2021), 2021 | Nigeria | CS | 43.8 | 50(70.00%) | Selenium | Kit | 21(29.1 ± 1.9) vs 50(25.3 ± 2.4) ng/dL | NA | NA |
| Bego et al. (2022), 2022 | Bosnia and Herzegovina | Pro | NA | 210(59.5%) | Selenium | ICP-MS | NA | NA vs 52(64.7 ± 19.8) µg/L | 51(85.9 ± 18.0) vs 53 (74.7 ± 18.0) µg/L |
| Kocak et al. (2022), 2021 | Turkey | Pro | 48.8 | 60(53.33%) | Selenium | ICP-MS | 32(255.23 ± 42.67) vs NA µg/L | NA | 15(196.85 ± 41.04) vs 13(206.97 ± 57.18) µg/L |
*Retro retrospective; Pro prospective; CS cross-section study;
**AAS atomic absorption spectrometer; ICP-MS inductively coupled plasma mass spectrometry; TXRF total reflection x-ray fluorescence;
***Mild and moderate cases were referred to as non-severe cases, and it represents mild if there are both mild and moderate
Association between Zinc/Selenium and severity in COVID-19.
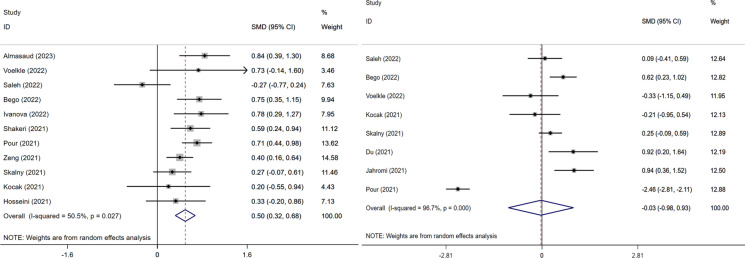
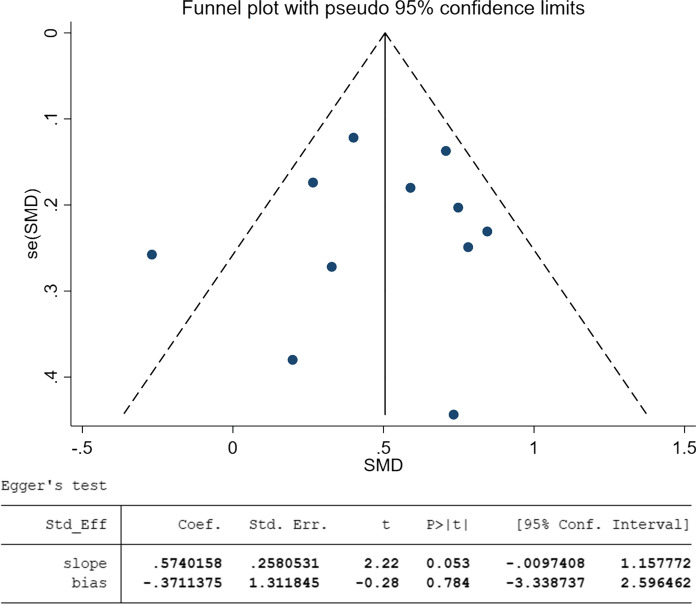
This meta-analysis of 11 studies showed that an elevated serum zinc was associated with an increased risk of severe COVID-19 (SMD = 0.50, 95%CI 0.32–0.68, I2 = 50.5%, Fig. 2). The funnel-plot was qualitatively asymmetrical for zinc. Regression-based Egger’s test showed no indication of small-study effects for zinc (p = 0.784) on the severity of disease (Fig. 3). However, analysis of 8 studies that elevated selenium level was not associated with an increased risk of severe COVID-19 (SMD = − 0.03, 95%CI − 0.98–0.93, I2 = 96.7%).
Fig. 2.
Forest plot of severity of COVID-19 disease of zinc and selenium divided into two groups based on the microelement. The weighted risk difference for individual trails was represented by the center of each square, and the accompanying horizontal line indicated the 95 percent CI. The diamonds indicated the aggregated results. And two pictures were named with: Left. The increased risk of severe COVID-19 disease with zinc; Right. The increased risk of severe COVID-19 disease with selenium
Fig. 3.
Funnel plot and Egger’s test on the severity of COVID-19 disease of zinc in the selected articles. And two pictures were named with: Above. Funnel plot with pseudo 95% confidence limits on zinc; Below. Egger’s test of zinc
Association between Zinc/Selenium and mortality in COVID-19
This meta-analysis of 3 studies showed that null association between serum zinc level and mortality in COVID-19 (SMD = 1.66, 95% CI − 1.42–4.47). Similarly, the meta-analysis of three studies also found that null association between serum selenium level and mortality in COVID-19 (SMD = -0.16, 95% CI − 1.33–1.01, Fig. 4).
Fig. 4.
Forest plot of mortality in COVID-19 disease of zinc and selenium divide into two groups based on the microelement. The weighted risk difference for individual trials is represented by the center of each square, and the accompanying horizontal line indicates the 95 percent CI. The diamonds indicate the aggregated results. And two pictures were named with: Left. The mortality of COVID-19 disease with zinc; Right. The mortality of COVID-19 disease with selenium
Association between Zinc/Selenium and risk of COVID-19 infection.
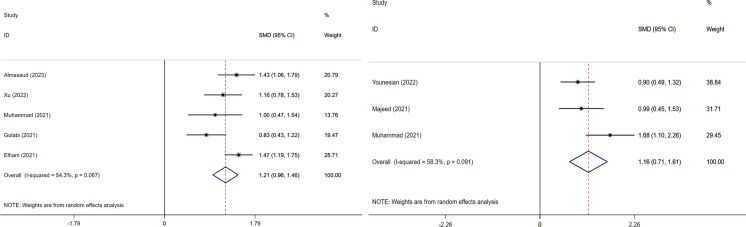
5 studied showed that serum zinc level was notable in COVID-19 patients (SMD = 1.21, 95% CI: 0.96–1.46, I2 = 54.3%). Similarly, three studies showed that serum selenium level was obvious in COVID-19 patients (SMD = 1.16, 95% CI: 0.71–1.61, I2 = 58.3%, Fig. 5).
Fig. 5.
Forest plot of risk in COVID-19 disease of zinc and selenium divide into two groups based on the microelement. The weighted risk difference for individual trials is represented by the center of each square, and the accompanying horizontal line indicates the 95 percent CI. The diamonds indicate the aggregated results. And two pictures were named with: Left. The risk of COVID-19 disease with zinc; Right. The risk of COVID-19 disease with selenium
Discussion
Role of zinc and selenium and effect on the COVID-19.
In response to viral infections, zinc and selenium act in collaboration. By modifying the proteolytic process of replicating (Pour et al. 2021; Kieliszek and Lipinski 2020), RNA-dependent RNA polymerase, and lowering RNA synthesis activity in viral illnesses (Hosseini et al. 2021), zinc prohibits viruses from reproducing polyproteins. Additionally, the upregulation of alpha interferon (IFN-α) production in infected cells through janus kinase/signal transducer and activator of transcription 1 (JAK/STAT1) cells can limit the signaling pathway and its enhancement of antiviral activity under the control of zinc's regulation of antiviral immunity. Selenium, in this situation, is a crucial part of several enzymes. And along with vitamin E, selenium suppresses the creation of free radicals (Kieliszek and Lipinski 2020). Lack of selenium impairs immune system performance and speeds up virus multiplication and mutation (Xu et al. 2022; Keshavarzi et al. 2012). Natural killer (NK) cell activity and CD4+ T and B cell function are both improved by selenium (Golabi et al. 2021).
Golabi's investigations (Malavolta et al. 2015; Tanumihardjo et al. 2016) revealed a substantial negative association between serum zinc levels and illness severity in the context of the function of zinc in response to COVID-19, which is generally consistent with our findings. Additionally, SARS-CoV-2-infected cells accelerated the propagation of the virus in an in vitro experiment employing Vero E6 cells in the presence of serum zinc levels below 50 g/dl (Hosseini et al. 2021; Nouarie et al. 2004). Our findings were supported by the findings (Im et al. 2020; Derwand et al. 2020) that, regardless of how mild or severe their disease was, all COVID-19 patients displayed symptoms identical to hypozincemia, and that blood zinc levels in patients were 8% lower than those in the general population (Pourbagheri-Sigaroodi et al. 2020). One study (Hoang and Han 2020) demonstrated that the pre-onset zinc level and the hypoglycemic response during infection are what determine the serum zinc ion concentration in patients infected with the virus. This finding correlates with the prevalence of SARS-CoV-2 affected by zinc deficiency in our study. According to other research (Moghaddam et al. 2020; Laing et al. 2021; Shang et al. 2020), zinc might somewhat decrease the SARS-CoV-2 virus's ability to replicate and, as a result, its viral activity. This finding raises the possibility that zinc, in conjunction with existing medications, could be used as a treatment for COVID-19 patients. Moreover, in a randomized controlled trial by Patel et al. (2021), high doses of intravenous zinc reversed the symptoms of zinc deficiency in the acute phase of COVID-19. This provided a deeper implication. However, in other clinical trials (Patel et al. 2021; Abd-Elsalam et al. 2021; Thomas et al. 2021), the positive effect of zinc supplement and its ion carrier hydroxychloroquine on COVID-19 patients was weak or almost lost its effect. This led to the prevention and repair role of zinc in COVID-19 patients unclear.
Metabolism of selenium and selenoproteins may improve the outcome of SARS-CoV-2 infection by reducing virus-induced oxidative stress, excessive inflammatory response and immune system dysfunction (Zhang et al. 2020a). There was a correlation between serum selenium status and COVID-19 cure rates (Shakeri et al. 2022; Wintergerst et al. 2006), particularly in individuals with inadequate or low selenium intakes (Al-Saleh et al. 2022). More than that, one study (Gammoh and Rink 2017) has revealed a significant frequency of thrombotic problems in patients with COVID-19 disease. Selenium regulates the production of pro-inflammatory components of thromboxane A2 (TXA2) and lipoxygenase, which in turn affects the arachidonic acid pathway. Furthermore, selenium status was positively associated with survival in patients infected with COVID-19 compared to patients not infected with COVID-19 (Kieliszek 2023). Moreover, selenium levels have reduced in COVID-19 patients worldwide (Zhang et al. 2020b; Rataan et al. 2022), suggesting a relationship between selenium deficiency and COVID-19 infection. Additionally, low C-reactive protein (CRP), neutrophil count to neutrophil to lymphocyte ratio (NLR), and lymphocyte and monocyte ratio (LMR) were associated with elevated selenium levels (Younesian et al. 2022; Schroeder and Cousins 1990; Shamblott et al. 1998), implying that selenium has an anti-inflammatory effect, particularly during viral infections, and also implying a positive correlation between selenium deficiency and the incidence of COVID-19 relationship. However, when modifying models for certain inflammatory indicators, these correlations were favorably changed (Carlucci et al. 2020; Skalny et al. 2020), indicating that selenium is independently linked with COVID-19 disease severity. Furthermore, the association of excess selenium intake with cure rates for COVID-19 has been demonstrated, although selenium intake to toxic levels is not recommended for selenium-sufficient individuals (Rayman 2012).
Heterogeneity and limitations
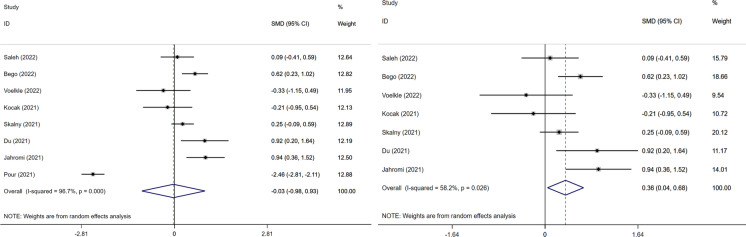
Utilizing a subgroup analysis to reduce the heterogeneity, we performed meta regression with disease extent (healthy vs patient; recovered vs dead; non-severe vs severe) as the subject compared with others and obtained relevant images. We considered that Pour's article might be a source of heterogeneity in our article, possibly due to the different inclusion measures of the data or to the intake of micronutrient supplements by the patients (Gouda et al. 2021; Hoffmann and Berry 2008), although he was consistent in terms of zinc. To obtain more precise results, we performed a sensitivity analysis involving selenium. It found that the removal of Pour’s article changed from SMD ( 0.03, 95% CI: 0.98–0.93) to SMD (0.36, 95% CI: 0.04–0.68) in terms of the severity of selenium and COVID-19, and started to show a positive correlation (Fig. 6), which coincided with the findings of Gombart et al. (2020). In addition, after we adopted NOS, each article scored above 5, which classified as high-quality articles.
Fig. 6.
Forest plot of severity of COVID-19 disease of selenium compared between two groups. And two pictures were named with: Left. The increased risk of severe COVID-19 disease with selenium; Right. The increased risk of severe COVID-19 disease with selenium with one research removing
At limitations, this article was limited by the amount of available literature and the way in which it was investigated, among others. Moreover, based on the current studies, very few studies were available to confirm the therapeutic effect of zinc. In the case of selenium, few articles were enough to demonstrate its clinical efficacy. Although we tried to analyze the effects of two serum micronutrient deficiencies on COVID-19, the possible role of these micronutrients for the organism remained more controversial (Balboni et al. 2022). This was possibly because the influence of the subject's own nutritional status, environmental and age factors can be equally limiting conditions when supplementing and testing for these two elements. Therefore, preventive or therapeutic interventions targeting COVID-19 based on zinc or selenium supplementation were currently not justified.
Conclusion
The meta-analysis showed that the correlation between zinc and selenium deficiency and the degree of risk for COVID-19 held, and zinc deficiency showed a higher correlation with the degree of progression of COVID-19. Nevertheless, due to the lack of randomized controlled trials, our conclusions need to be confirmed by randomized controlled trials with larger numbers of subjects.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
None.
Author contributions
QX conceived ideas, analyzed data; LF drafted the manuscript, contributed towards the conception; YC, ZL, JG, XG, YZ, WT, and JZ made great efforts to polish and revise the manuscript. All the authors provided critical review and approved the final manuscript before submission.
Funding
This work was supported by National Innovation and Entrepreneurship Training Program for College Students (No. 202110443046), the Research Fund for Lin He’s Academician Workstation of New Medicine and Clinical Translation in Jining Medical University(JYHL2019ZD03), the Shandong Medical and Health Technology Development Plan Project of Shandong Province (2017WS339), and the Science and Technology Project of Colleges in Shangdong Province (J17KB085, J18KA267).
Data availability
The datasets generated during and/or analysed during the current study are available in the PubMed, Embase and, WOS repository.
Declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical Approval
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abd-Elsalam S, Soliman S, Esmail ES, Khalaf M, Mostafa EF, Medhat MA, Ahmed OA, El Ghafar MSA, Alboraie M, Hassany SM. Do zinc supplements enhance the clinical efficacy of hydroxychloroquine?: A randomized multicenter trial. Biol Trace Elem Res. 2021;199(10):3642–3646. doi: 10.1007/s12011-020-02512-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Almasaud AS, Chalabi J, Arfaj AA, Qarni AA, Alkroud A, Nagoor Z, Akhtar S, Iqbal J. Association of serum zinc and inflammatory markers with the severity of covid-19 infection in adult patients. Nutrients. 2023;15(2):340. doi: 10.3390/nu15020340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Saleh I, Alrushud N, Alnuwaysir H, Elkhatib R, Shoukri M, Aldayel F, Bakheet R, Almozaini M. Essential metals, vitamins and antioxidant enzyme activities in COVID-19 patients and their potential associations with the disease severity. Biometals. 2022;35(1):125–145. doi: 10.1007/s10534-021-00355-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balboni E, Zagnoli F, Filippini T, Fairweather-Tait SJ, Vinceti M. Zinc and selenium supplementation in COVID-19 prevention and treatment: a systematic review of the experimental studies. J Trace Elem Med Biol. 2022;71:126956. doi: 10.1016/j.jtemb.2022.126956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bego T, Meseldžić N, Prnjavorac B, Prnjavorac L, Marjanović D, Azevedo R, Pinto E, Duro M, Couto C, Almeida A. Association of trace element status in COVID-19 patients with disease severity. J Trace Elem Med Biol. 2022;74:127055. doi: 10.1016/j.jtemb.2022.127055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlucci PM, Ahuja T, Petrilli C, Rajagopalan H, Jones S, Rahimian J. Zinc sulfate in combination with a zinc ionophore may improve outcomes in hospitalized COVID-19 patients. J Med Microbiol. 2020;69(10):1228–1234. doi: 10.1099/jmm.0.001250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derwand R, Scholz M, Zelenko V. COVID-19 outpatients: early risk-stratified treatment with zinc plus low-dose hydroxychloroquine and azithromycin: a retrospective case series study. Int J Antimicrob Agents. 2020;56(6):106214. doi: 10.1016/j.ijantimicag.2020.106214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Laing G, Petrovic M, Lachat C, De Boevre M, Klingenberg GJ, Sun Q, De Saeger S, De Clercq J, Ide L, Vandekerckhove L, Schomburg L. Course and survival of COVID-19 patients with comorbidities in relation to the trace element status at hospital admission. Nutrients. 2021;13(10):3304. doi: 10.3390/nu13103304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elham AS, Azam K, Azam J, Mostafa L, Nasrin B, Marzieh N. Serum vitamin D, calcium, and zinc levels in patients with COVID-19. Clin Nutr ESPEN. 2021;43:276–282. doi: 10.1016/j.clnesp.2021.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammoh NZ, Rink L. Zinc in infection and inflammation. Nutrients. 2017;9(6):624. doi: 10.3390/nu9060624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golabi S, Adelipour M, Mobarak S, Piri M, Seyedtabib M, Bagheri R, Suzuki K, Ashtary-Larky D, Maghsoudi F, Naghashpour M. The association between vitamin D and zinc status and the progression of clinical symptoms among outpatients infected with SARS-CoV-2 and potentially non-infected participants: a cross-sectional study. Nutrients. 2021;13(10):3368. doi: 10.3390/nu13103368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombart AF, Pierre A, Maggini S. A review of micronutrients and the immune system-working in harmony to reduce the risk of infection. Nutrients. 2020;12(1):236. doi: 10.3390/nu12010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouda AS, Adbelruhman FG, Elbendary RN, Alharbi FA, Alhamrani SQ, Mégarbane B. A comprehensive insight into the role of zinc deficiency in the renin-angiotensin and kinin-kallikrein system dysfunctions in COVID-19 patients. Saudi J Biol Sci. 2021;28(6):3540–3547. doi: 10.1016/j.sjbs.2021.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang BX, Han B. A possible application of hinokitiol as a natural zinc ionophore and anti-infective agent for the prevention and treatment of COVID-19 and viral infections. Med Hypotheses. 2020;145:110333. doi: 10.1016/j.mehy.2020.110333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann PR, Berry MJ. The influence of selenium on immune responses. Mol Nutr Food Res. 2008;52(11):1273–1280. doi: 10.1002/mnfr.200700330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini SJ, Moradi B, Marhemati M, Firouzian AA, Ildarabadi E, Abedi A, Firooz M. Comparing serum levels of vitamin d and zinc in novel coronavirus-infected patients and healthy individuals in northeastern Iran, 2020. Infect Dis Clin Pract. 2021;29(6):e390–e394. doi: 10.1097/IPC.0000000000001051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im JH, Je YS, Baek J, Chung MH, Kwon HY, Lee JS. Nutritional status of patients with COVID-19. Int J Infect Dis. 2020;100:390–393. doi: 10.1016/j.ijid.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova ID, Pal A, Simonelli I, Atanasova B, Ventriglia M, Rongioletti M, Squitti R. Evaluation of zinc, copper, and Cu: Zn ratio in serum, and their implications in the course of COVID-19. J Trace Elem Med Biol. 2022;71:126944. doi: 10.1016/j.jtemb.2022.126944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahromi SR, Tabriz HM, Togha M, et al. The correlation between serum selenium, zinc, and COVID-19 severity: an observational study. BMC Infect Dis. 2021;21:899. doi: 10.1186/s12879-021-06617-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayawardena R, Sooriyaarachchi P, Chourdakis M, Jeewandara C, Ranasinghe P. Enhancing immunity in viral infections, with special emphasis on COVID-19: a review. Diabetes Metab Syndr. 2020;14(4):367–382. doi: 10.1016/j.dsx.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavarzi B, Moore F, Najmeddin A, Rahmani F, Malekzadeh A. Quality of drinking water and high incidence rate of esophageal cancer in Golestan province of Iran: a probable link. Environ Geochem Health. 2012;34(1):15–26. doi: 10.1007/s10653-011-9377-3. [DOI] [PubMed] [Google Scholar]
- Kieliszek M. Selenium in the prevention of SARS-CoV-2 and other viruses. Biol Trace Elem Res. 2023;201(2):655–662. doi: 10.1007/s12011-022-03208-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieliszek M, Lipinski B. Selenium supplementation in the prevention of coronavirus infections (COVID-19) Med Hypotheses. 2020;143:109878. doi: 10.1016/j.mehy.2020.109878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocak OF, Ozgeris FB, Parlak E, Kadıoglu Y, Yuce N, Yaman ME, Bakan E. Evaluation of serum trace element levels and biochemical parameters of COVID-19 patients according to disease severity. Biol Trace Elem Res. 2022;200(7):3138–3146. doi: 10.1007/s12011-021-02946-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27(6):1785–1805. doi: 10.1177/0962280216669183. [DOI] [PubMed] [Google Scholar]
- Majeed M, Nagabhushanam K, Gowda S, Mundkur L. An exploratory study of selenium status in healthy individuals and in patients with COVID-19 in a south Indian population: the case for adequate selenium status. Nutrition. 2021;82:111053. doi: 10.1016/j.nut.2020.111053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malavolta M, Piacenza F, Basso A, Giacconi R, Costarelli L, Mocchegiani E. Serum copper to zinc ratio: relationship with aging and health status. Mech Ageing Dev. 2015;151:93–100. doi: 10.1016/j.mad.2015.01.004. [DOI] [PubMed] [Google Scholar]
- McGuinness LA, Higgins JP. Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Research Synthesis Methods. 2020;12:55–61. doi: 10.1002/jrsm.1411. [DOI] [PubMed] [Google Scholar]
- Moghaddam A, Heller RA, Sun Q, Seelig J, Cherkezov A, Seibert L, Hackler J, Seemann P, Diegmann J, Pilz M, Bachmann M, Minich WB, Schomburg L. Selenium deficiency is associated with mortality risk from COVID-19. Nutrients. 2020;12(7):2098. doi: 10.3390/nu12072098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad Y, Kani YA, Iliya S, Muhammad JB, Binji A, El-Fulaty Ahmad A, Kabir MB, Umar Bindawa K, Ahmed A. Deficiency of antioxidants and increased oxidative stress in COVID-19 patients: a cross-sectional comparative study in Jigawa. Northwestern Nigeria SAGE Open Med. 2021 doi: 10.1177/2050312121991246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouarie M, Pourshams A, Kamangar F, Sotoudeh M, Derakhshan MH, Akbari MR, Fakheri H, Zahedi MJ, Caldwell K, Abnet CC, Taylor PR, Malekzadeh R, Dawsey SM. Ecologic study of serum selenium and upper gastrointestinal cancers in Iran. World J Gastroentero. 2004;10(17):2544–2546. doi: 10.3748/wjg.v10.i17.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel O, Chinni V, El-Khoury J, Perera M, Neto AS, McDonald C, See E, Jones D, Bolton D, Bellomo R, Trubiano J, Ischia J. A pilot double-blind safety and feasibility randomized controlled trial of high-dose intravenous zinc in hospitalized COVID-19 patients. J Med Virol. 2021;93(5):3261–3267. doi: 10.1002/jmv.26895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pour OB, Yahyavi Y, Karimi A, Khamaneh AM, Milani M, Khalili M, Sharifi A. Serum trace elements levels and clinical outcomes among Iranian COVID-19 patients. Int J Infect Dis. 2021;111:164–168. doi: 10.1016/j.ijid.2021.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourbagheri-Sigaroodi A, Bashash D, Fateh F, Abolghasemi H. Laboratory findings in COVID-19 diagnosis and prognosis. Clin Chim Acta. 2020;510:475–482. doi: 10.1016/j.cca.2020.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rataan AO, Geary SM, Zakharia Y, Rustum YM, Salem AK. Potential role of selenium in the treatment of cancer and viral infections. Int J Mol Sci. 2022;23(4):2215. doi: 10.3390/ijms23042215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayman MP. Selenium and human health. Lancet. 2012;379:9822. doi: 10.1016/S0140-6736(11)61452-9. [DOI] [PubMed] [Google Scholar]
- Schroeder JJ, Cousins RJ. Interleukin 6 regulates metallothionein gene expression and zinc metabolism in hepatocyte monolayer cultures. Proc Natl Acad Sci U S A. 1990;87(8):3137–3141. doi: 10.1073/pnas.87.8.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakeri H, Azimian A, Ghasemzadeh-Moghaddam H, Safdari M, Haresabadi M, Daneshmand T, Namdar Ahmadabad H. Evaluation of the relationship between serum levels of zinc, vitamin B12, vitamin D, and clinical outcomes in patients with COVID-19. J Med Virol. 2022;94(1):141–146. doi: 10.1002/jmv.27277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamblott MJ, Axelman J, Wang S, Bugg EM, Littlefield JW, Donovan PJ, Blumenthal PD, Huggins GR, Gearhart JD. Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc Natl Acad Sci USA. 1998;95(23):13726–13731. doi: 10.1073/pnas.95.23.13726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang W, Dong J, Ren Y, Tian M, Li W, Hu J, Li Y. The value of clinical parameters in predicting the severity of COVID-19. J Med Virol. 2020;92(10):2188–2192. doi: 10.1002/jmv.26031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalny AV, Rink L, Ajsuvakova OP, Aschner M, Gritsenko VA, Alekseenko SI, Svistunov AA, Petrakis D, Spandidos DA, Aaseth J, Tsatsakis A, Tinkov AA. Zinc and respiratory tract infections: perspectives for COVID-19 (Review) Int J Mol Med. 2020;46(1):17–26. doi: 10.3892/ijmm.2020.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalny AV, Timashev PS, Aschner M, Aaseth J, Chernova LN, Belyaev VE, Grabeklis AR, Notova SV, Lobinski R, Tsatsakis A, Svistunov AA, Fomin VV, Tinkov AA, Glybochko PV. Serum zinc, copper, and other biometals are associated with COVID-19 severity markers. Metabolites. 2021;11(4):244. doi: 10.3390/metabo11040244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanumihardjo SA, Russell RM, Stephensen CB, Gannon BM, Craft NE, Haskell MJ, Lietz G, Schulze K, Raiten DJ. Biomarkers of Nutrition for Development (BOND)—Vitamin a review. J Nutr. 2016;146:9. doi: 10.3945/jn.115.229708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S, Patel D, Bittel B, Wolski K, Wang Q, Kumar A, Il'Giovine ZJ, Mehra R, McWilliams C, Nissen SE, Desai MY. Effect of high-dose zinc and ascorbic acid supplementation vs usual care on symptom length and reduction among ambulatory patients with SARS-CoV-2 infection: the COVID A to Z randomized clinical trial. JAMA Netw Open. 2021;4(2):e210369. doi: 10.1001/jamanetworkopen.2021.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelkle M, Gregoriano C, Neyer P, Koch D, Kutz A, Bernasconi L, Conen A, Mueller B, Schuetz P. Prevalence of micronutrient deficiencies in patients hospitalized with COVID-19: an observational cohort study. Nutrients. 2022;14(9):1862. doi: 10.3390/nu14091862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels I, Rolles B, Rink L. The potential impact of zinc supplementation on COVID-19 pathogenesis. Front Immunol. 2020;11:1712. doi: 10.3389/fimmu.2020.01712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels I, Rolles B, Slusarenko AJ, Rink L. Zinc deficiency as a possible risk factor for increased susceptibility and severe progression of corona virus disease 19. Br J Nutr. 2022;127(2):214–232. doi: 10.1017/S0007114521000738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintergerst ES, Maggini S, Hornig DH. Immune-enhancing role of vitamin C and zinc and effect on clinical conditions. Ann Nutr Metab. 2006;50(2):85–94. doi: 10.1159/000090495. [DOI] [PubMed] [Google Scholar]
- Xu W, Liu Y, Zou X, Luo H, Wu W, Xia J, Chan MTV, Fang S, Shu Y, Wu WKK, Zhang L. Hypozincemia in COVID-19 patients correlates with stronger antibody response. Front Immunol. 2022;12:785599. doi: 10.3389/fimmu.2021.785599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui Y, Yasui H, Suzuki K, Saitou T, Yamamoto Y, Ishizaka T, Nishida K, Yoshihara S, Gohma I, Ogawa Y. Analysis of the predictive factors for a critical illness of COVID-19 during treatment—Relationship between serum zinc level and critical illness of COVID-19. Int J Infect Dis. 2020;100:230–236. doi: 10.1016/j.ijid.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younesian O, Khodabakhshi B, Abdolahi N, Norouzi A, Behnampour N, Hosseinzadeh S, Alarzi SSH, Joshaghani H. Decreased serum selenium levels of covid-19 patients in comparison with healthy individuals. Biol Trace Elem Res. 2022;200(4):1562–1567. doi: 10.1007/s12011-021-02797-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng HL, Yang Q, Yuan P, Wang X, Cheng L. Associations of essential and toxic metals/metalloids in whole blood with both disease severity and mortality in patients with COVID-19. FASEB J. 2021;35(3):e21392. doi: 10.1096/fj.202002346RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Saad R, Taylor EW, Rayman MP. Selenium and selenoproteins in viral infection with potential relevance to COVID-19. Redox Biol. 2020;2020(37):101715. doi: 10.1016/j.redox.2020.101715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Taylor EW, Bennett K, Saad R, Rayman MP. Association between regional selenium status and reported outcome of COVID-19 cases in China. Am J Clin Nutr. 2020;111(6):1297–1299. doi: 10.1093/ajcn/nqaa095. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available in the PubMed, Embase and, WOS repository.