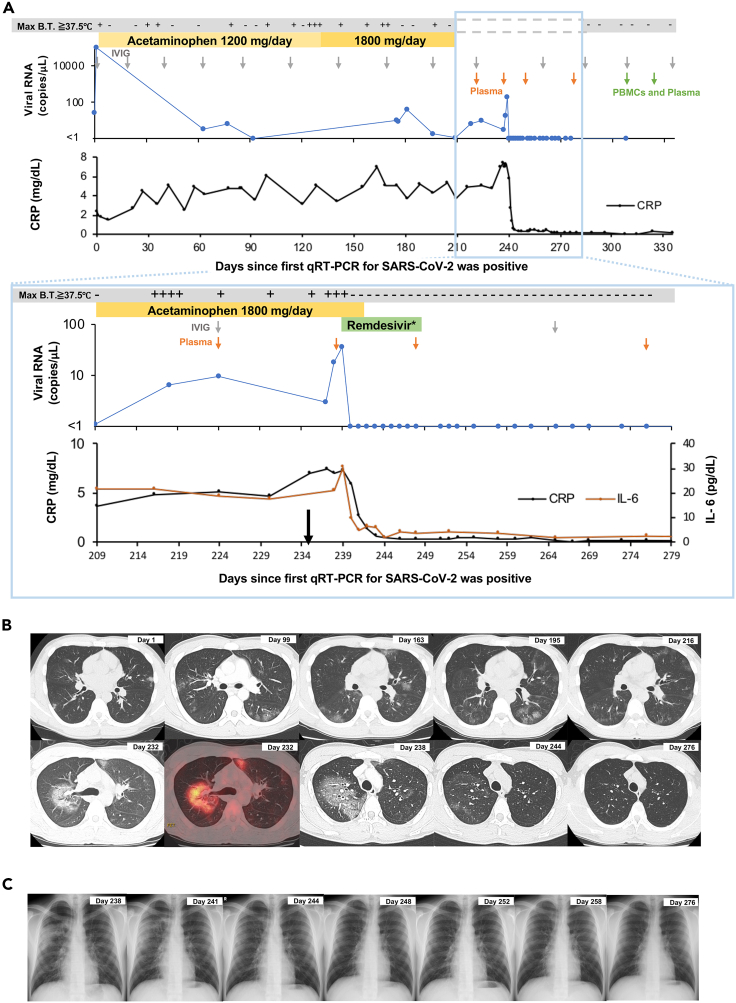
Figure 1.
Clinical and laboratory data from the patient during the observation period
(A) Results of qRT-PCR for SARS-CoV-2; days in which there was a maximum body temperature above 37.5°C; and blood tests during the observation period. The black arrow indicates the time of symptom exacerbation. The gray arrow indicates the administration time of intravenous immunoglobulin (IVIG). The green and orange arrows indicate the collection time of PBMCs for the T-cell analysis and/or plasma for the measurement of the anti-SARS-CoV-2 spike antibody, respectively. qRT-PCR, real-time quantitative reverse transcriptase polymerase chain reaction; Max B.T., maximum body temperature of the day. ∗ Remdesivir was administered at 200 mg/day on the first day and 100 mg/day for the following 9 days.
(B) Computed tomography (black and white images) and positron emission tomography/computed tomography (colored image) results during the observation period.
(C) Chest X-ray results during the observation period, particularly after starting the administration of remdesivir. See also Tables S1–S3 and S5.