Abstract
Objectives:
Assess degree to which US parents are likely to have their children get COVID-19 vaccines and identify parental concerns about the vaccines.
Methods:
In February – March 2021, we surveyed parent members of a nationally representative probability-based internet panel of ≈9,000 adults regarding their intent to have their children receive a COVID-19 vaccination, perceptions of COVID-19 vaccines for children, and trust in sources of information about COVID-19 vaccines for children. We used descriptive and multivariate analyses to evaluate parent-stated likelihood of having their children get a COVID-19 vaccine and to assess the association between likelihood of child COVID-19 vaccination and child age, parent demographics, and parental perceptions about COVID-19 vaccines.
Results:
Altogether, 1,745 parents responded (87% of eligible parents, 3,759 children). Likelihood of child COVID-19 vaccination was as follows: very likely (28%), somewhat likely (18%), somewhat unlikely (9%), very unlikely (33%), and unsure (12%). The stated likelihood of child vaccination was greater among parents of older children (p<.001) as well as parents who had Bachelor’s or higher education (p<.001), had already received or were likely to receive a COVID-19 vaccine (p<.001), or had Democratic affiliation (p<.001); variations existed by race/ethnicity (p=.04). Parental concerns centered around vaccine safety and side-effects. A key trusted source of information about COVID-19 vaccines for children was the child’s doctor.
Conclusions
Less than half of US participants report that they are likely to have their child receive a COVID-19 vaccine. Pediatric healthcare providers have a major role in promoting and giving COVID-19 vaccination for children.
Table of Contents Summary
Less than half of US participants report that they are likely to have their child receive a COVID-19 vaccine.
Article Summary
This nationally representative survey found that less than one-half of US parents are likely to have their child receive COVID-19 vaccines when they are available.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has affected children and adolescents and children now account for more than one-fifth of new cases in the United States (US).1 Although most pediatric infections are asymptomatic or mild, many children have been hospitalized, more than 300 have died from related complications, and thousands have suffered from Multisystem Inflammatory Syndrome in Children (MIS-C).1 Children have experienced stress from online schooling, disrupted health and social services, family illnesses, and social isolation,2 with a disproportionate toll among low-income and racial/ethnic minority children.3
As 22% (74 million) of the US population is under 18 years, COVID-19 vaccines for children are needed for both direct and community protection.4 Currently three vaccines are recommended by the Advisory Committee on Immunization Practices (ACIP) and the Centers for Disease Control (CDC);5–7 the Pfizer vaccine was recommended for individuals over age 16 years in December 20206 and recently authorized for children 12–15 years.8 Trials are underway to assess the safety, reactogenicity, immunogenicity, and effectiveness of COVID-19 vaccines in younger children.9,10 However, these vaccines can only be effective if parents desire them.
Vaccine hesitancy is a major public health concern worldwide.11,12 About 6% of US parents are hesitant about routine childhood vaccines other than influenza vaccine,13 26% are hesitant about influenza vaccine,13 and 23% are hesitant about HPV vaccine.14 Parental concerns center around questionable seriousness of the infections, vaccine effectiveness, and vaccine side effects;13,14 these issues are relevant for COVID-19 vaccines15 as well.
Although much is known about COVID-19 vaccine hesitancy among adults,16–20 little is known about parental hesitancy for COVID-19 vaccines for children.16,21–23 Rhodes21 surveyed US parents of preschool children who were pre-screened as hesitant toward childhood vaccines, noting high hesitancy about a future COVID-19 vaccine for children; parents desired information from healthcare professionals and alternative medical providers. An early study in April 2020 noted high intent to vaccinate.24,25
Our study objectives were to assess the willingness of US parents to have their children get a pediatric COVID-19 vaccine and to identify parental concerns about the vaccines. Between February 17 – March 30, 2021, we surveyed a representative online sample of US parents about their perceptions regarding COVID-19 vaccines for their children (once available). We also assessed levels of trust in pediatric providers and other sources of information about COVID-19 vaccines for children.
Methods
The University of Southern California’s IRB approved the study. Participants provided written, informed consent.
The Understanding America Study (UAS)
The UAS is a probability-based internet panel of about 9,000 adults 18 years of age and over, which is representative of the non-institutionalized US population.26 Panel members are recruited using address-based sampling, and tablets and broadband internet are provided if needed. Surveys are in English or Spanish. Between April 1, 2020 and February 16, 2021, the UAS surveyed subsamples of the online panel every two weeks about the COVID-19 pandemic;16,26 starting February 17, 2021, surveys are administered monthly. Survey invitations are balanced by age, sex, employment status, and Los Angeles County residence (these residents were oversampled in the UAS panel). General UAS weights take into account respondents’ gender, race/ethnicity, education, and Census regions, while also correcting for the Los Angeles County oversample. We analyzed the survey sent February 17 – March 16; panelists had 14 days to respond to the survey so that the survey period was February 17 – March 30. Incentives were provided to encourage survey completion, including an additional incentive to complete the survey on the day respondents receive their invitation. Over 90% of responses were received within the first 4 weeks of fielding this survey.
Questionnaire Development
We adapted questions from prior surveys14,16 and added new questions as a new 3-minute survey module on COVID-19 vaccines for children. Respondents received an additional $2 to complete the module. Questions regarding parental intent and plans to vaccinate their children were asked about each child in the household. All additional child-specific questions were asked about each child in the household for parents of up to three children; for parents of four or more children, three children were a randomly selected from the household.
Demographic Measures:
The UAS has detailed information about the demographic characteristics of panelists, which is updated quarterly. Parental demographic factors included parental age, sex, race/ethnicity, and education level.
Intent to have the child get COVID-19 Vaccine:
We asked: “How likely are you to get [Child’s name] vaccinated for coronavirus once a vaccine is available for children?” [very likely, somewhat likely, somewhat unlikely, very unlikely, or unsure]. We coded “very likely or somewhat likely” as “likely to get a coronavirus vaccine”; all others were labelled as “hesitant.” We also asked: “If a vaccine against the coronavirus becomes available for children, do you plan to get [Child’s name] vaccinated”? [yes as soon as possible, yes but I want to wait and see, no but I want to wait and see, no I will not get a coronavirus vaccine for my child, and not sure].
Perceptions about COVID-19 vaccines for children (Table 3):
Table 3.
Percent of parents who strongly or somewhat agree with each of these statements, by whether they are very/somewhat likely or very/somewhat unlikely to have their children vaccinated against COVID-19.
| Percent of parents who strongly agree or agree with statements* | ||
|---|---|---|
| Very/Somewhat Likely to Vaccinate Child | Very/Somewhat Unlikely/Unsure To Vaccinate Child | |
| Positive perceptions about COVID-19 vaccines | ||
| A COVID-19 vaccine will be important for [NAME]’s health. | 93.8% | 27.1% |
| Getting a COVID-19 vaccine would be a good way to protect [NAME] from coronavirus disease. | 96.0% | 32.2% |
| A COVID-19 vaccine will be effective if it is approved by the FDA or CDC. | 95.8% | 40.9% |
| Getting a COVID-19 vaccine will be important for the health of others in my community. | 96.2% | 43.0% |
| A COVID-19 vaccine will be beneficial to [NAME]. | 95.3% | 29.4% |
| I will do what [NAME]’s doctor or health care provider recommends about a COVID-19 vaccine. | 94.7% | 41.5% |
| Negative perceptions about COVID-19 vaccines | ||
| A COVID-19 vaccine will not have been around long enough to be sure it is safe. | 47.7% | 81.6% |
| I am concerned about serious side effects of a COVID-19 vaccine. | 55.1% | 86.4% |
| I think a COVID-19 vaccine might cause lasting health problems for [NAME]. | 26.8% | 78.0% |
The parent-child pair was the unit of analysis. Parents’ sampling weights were used in the analyses to account for design effects.
We modified questions from the World Health Organization’s Vaccine Hesitancy Scale (VHS) to apply to COVID-19 vaccines for children.27 The VHS assesses dimensions of vaccine confidence and risks, has been psychometrically validated, and has been used in in numerous countries to examine vaccine hesitancy.28–30 We previously modified VHS questions for routine childhood vaccines, pediatric influenza vaccine, and human papillomavirus (HPV) vaccine.13,14
Parental likelihood of getting a COVID-19 vaccine:
We asked parents about their own likelihood of getting a COVID-19 vaccine (same 5-point Likert scale); respondents who had already received a COVID-19 vaccine were coded as “very likely.”
Prior influenza vaccination:
We asked whether the child had received “a flu vaccine” in the past two influenza vaccination seasons (2019–2020 and 2020–2021).
Sources of trusted information about COVID-19 vaccine:
We asked: “How much do you trust the following sources of information about the coronavirus vaccine?” [do not trust at all, trust somewhat, trust mostly, trust completely, not applicable]. Sources were: child’s doctor; child’s school or school district; respondent’s local public health department; Centers for Disease Control and Prevention (CDC); American Academy of Pediatrics (AAP); respondent’s close friends and family members, coworkers, classmates, or other acquaintances; and social media (e.g., Facebook, Instagram, Twitter).
Trust in vaccine development and approval process:
We asked: “How much do you trust the process in general (not just for COVID-19) to develop safe vaccines for children? [fully trust, mostly trust, somewhat trust, do not trust]” and “How much do you trust the governmental approval process to ensure a COVID-19 vaccine is safe for children? [fully trust, mostly trust, somewhat trust, do not trust]”.
Analyses
We performed descriptive analyses to assess parents’ stated likelihood of having their child get a COVID-19 vaccine and plans to get a COVID-19 vaccine for their child. We assessed associations between vaccination likelihood and demographics, trust in different sources of information, and trust in the vaccine development and government approval processes.
To assess the association between parent-stated likelihood of getting their child a COVID-19 vaccine and above-mentioned factors, we used descriptive analyses and multivariable Poisson regression models with cluster-robust standard errors. For multivariate analyses, we included the following covariates in our primary model: parent factors (gender, age group, education, race/ethnicity, political party affiliation, and receipt of/likelihood to receive a COVID-19 vaccine) and child factors (age group and receipt of prior influenza vaccine).
Since a participating parent could report their vaccination intentions for multiple children, and since multiple parents from a given household could report their intentions for the same child, the unit of observation was parent-child pairs. Intention data were clustered at the household level and parent-level survey weights were used to make inferences about the US parent population. We use “% of parents” or “% children” as shorthand to summarize parent-level and child-level characteristics, respectively, as a percentage of the sampled parent-child pairs. We used a significance level of 0.05 for all analyses and conducted analyses using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
Altogether, 1,745 parents responded to the survey, and data are available for 3,759 children (Table 1); 33% of parents had high school or less education, and 56% were white, 22% Hispanic, 13% Black, and 5% Asian. Among children, 40% were 11 – 18 years of age, 36% were 5 – 10 years, and 24% were below 5 years.
Table 1:
Parents’ reported likelihood of getting their child vaccinated once a coronavirus vaccine is available for children.
| Likelihood of the child getting a coronavirus vaccination** | |||||||
|---|---|---|---|---|---|---|---|
| Weighted N | Very Likely | Somewhat Likely | Somewhat Unlikely | Very Unlikely | Unsure | P value | |
| Overall | 3,759 | 28.1% | 18.2% | 8.8% | 32.9% | 11.9% | - |
| Parent Characteristics | |||||||
| Parental Gender | |||||||
| Female | 2,177 | 23.1% | 18.5% | 8.6% | 36.2% | 13.5% | P=0.001 |
| Male | 1,582 | 34.9% | 17.7% | 9.2% | 28.5% | 9.7% | |
| Parental Age (years) | |||||||
| 18–39 | 2,319 | 23.3% | 17.7% | 10.1% | 37.9% | 11.0% | P<0.0001 |
| 40–49 | 870 | 34.3% | 20.1% | 6.6% | 27.6% | 11.4% | |
| 50+ | 570 | 38.2% | 17.2% | 7.3% | 20.6% | 16.8% | |
| Parental Education | |||||||
| High School or Less | 1,226 | 24.0% | 14.5% | 7.0% | 38.2% | 16.3% | P=0.0002 |
| Some College | 1,194 | 20.7% | 19.3% | 9.8% | 36.5% | 13.7% | |
| Bachelor’s or More | 1,337 | 38.3% | 20.6% | 9.7% | 25.0% | 6.3% | |
| Parental Race/Ethnicity | |||||||
| White | 843 | 25.5% | 20.0% | 7.0% | 36.5% | 11.0% | P<0.0366 |
| Hispanic | 2,089 | 35.4% | 16.4% | 11.2% | 24.4% | 12.7% | |
| Black | 489 | 22.1% | 11.7% | 9.0% | 37.8% | 19.3% | |
| Asian | 178 | 41.4% | 21.8% | 15.6% | 19.0% | 2.3% | |
| Other | 156 | 28.4% | 19.7% | 13.1% | 32.6% | 6.2% | |
| Parental Party Affiliation** | |||||||
| Democrat | 1,125 | 42.1% | 19.3% | 7.3% | 17.2% | 14.1% | P<0.0001 |
| Republican | 1,114 | 15.2% | 18.4% | 9.3% | 46.2% | 10.9% | |
| Other | 870 | 24.0% | 16.8% | 11.6% | 38.4% | 9.2% | |
| Parent received or likely to COVID vaccine | |||||||
| Yes | 2,087 | 47.9% | 27.3% | 9.0% | 8.0% | 7.8% | P<0.0001 |
| No | 1,672 | 3.2% | 6.8% | 8.7% | 64.2% | 17.1% | |
| Child Characteristics | |||||||
| Child’s Age (years) | |||||||
| 11–18 | 1,519 | 32.7% | 18.0% | 7.7% | 29.4% | 12.1% | P=0.0004 |
| 5–10 | 1,356 | 27.0% | 18.9% | 8.6% | 32.6% | 12.9% | |
| 0–4 | 884 | 21.8% | 17.4% | 11.1% | 39.5% | 10.2% | |
| Child received influenza vaccine (prior 2 years) | |||||||
| Yes | 1,722 | 41.5% | 24.5% | 9.0% | 16.6% | 8.4% | P<0.0001 |
| No | 1,760 | 15.0% | 11.8% | 8.8% | 49.5% | 14.8% | |
The parent-child pair was the unit of analysis. Parents’ sampling weights were used in the analyses to account for design effects.
Data are missing on party affiliation for 17% of parents.
For 46% of children, parents were “very likely” or “likely” to have their child get the vaccine, for 9% parents were “somewhat unlikely,” for 33% parents were “very unlikely” and for 12% parents were “unsure” (Table 1). Factors associated with higher likelihood of getting a COVID-19 vaccine for children included: parental Bachelor’s degree or higher educational attainment, parent being Asian or Latino, parents having already received or likely to receive a COVID-19 vaccine themselves, older age of child, and child having received an influenza vaccine in the past 2 years.
Figure 1 shows findings for whether parents plan to get their child vaccinated: 48% said “yes” (26% “yes ASAP”, 23% “yes but wait and see”), 39% said “no” (27% “no”, 12% “no but wait and see”), and 13% said “unsure.” Patterns were similar by child age and parent race/ethnicity.
Figure 1.
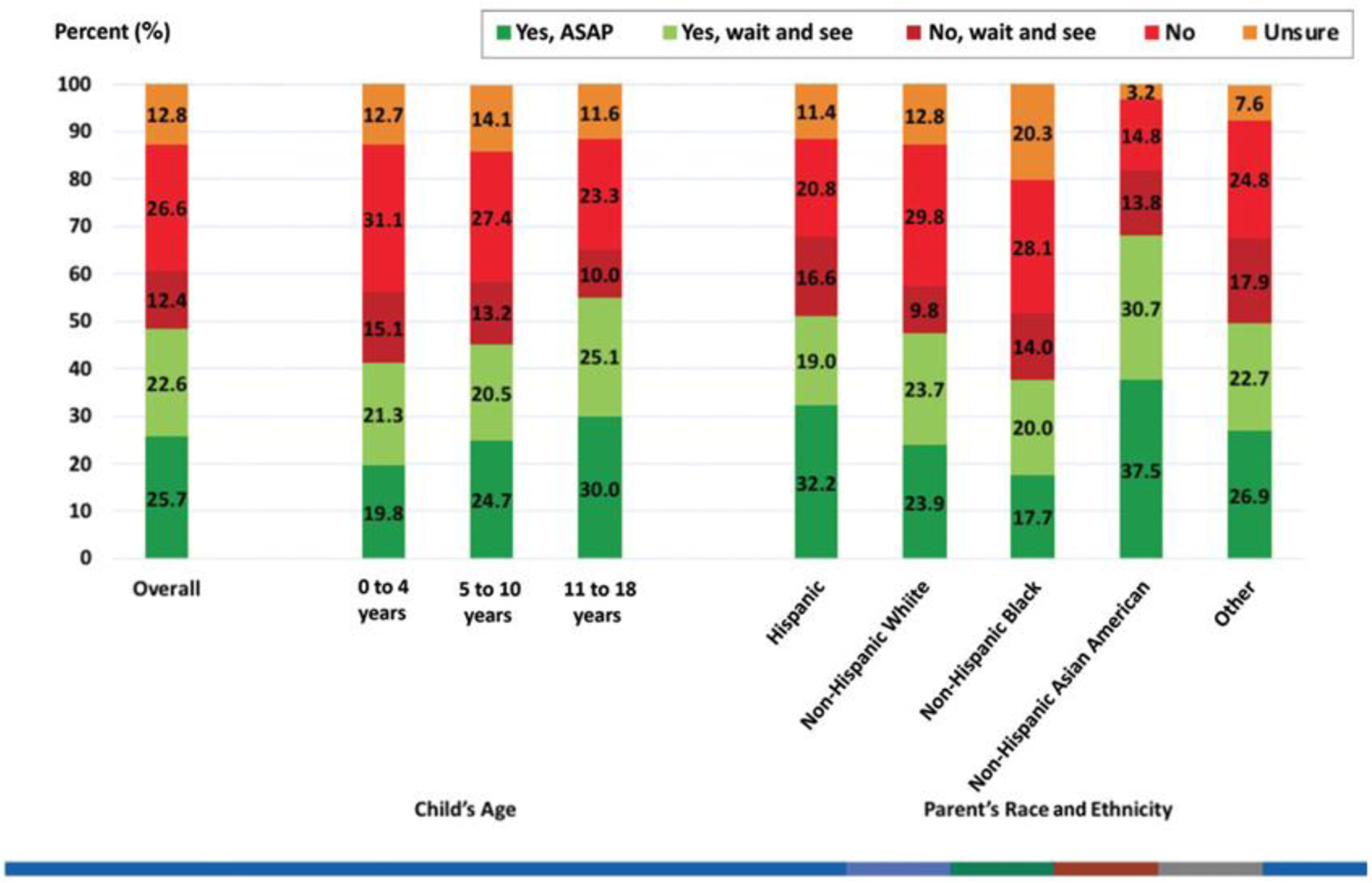
Parents’ response to the question: “If a vaccine against the coronavirus becomes available for children, do you plan to get [Child’s name] vaccinated”?
Among parents who had already received or are likely to get a COVID-19 vaccine (Table 2), 75% indicated they are “very likely” or “somewhat likely” to get the vaccine for their child; conversely, among parents who stated they are “unlikely,” “somewhat unlikely,” or “unsure” about the vaccine for themselves, only 10% responded they are very or somewhat likely to get their child vaccinated.
Table 2.
Parents’ intent to have their children vaccinated based upon their own COVID-19 vaccination.
| Parents very likely or likely to have their child get the COVID-19 vaccine* | |||
|---|---|---|---|
| Yes | No | ||
| Parents very likely or somewhat likely to get the COVID-19 vaccine for themselves | Yes | 75.2% | 24.8% |
| No | 10.0% | 90.0% | |
The parent-child pair was the unit of analysis. Parents’ sampling weights were used in the analyses to account for design effects.
A high proportion of parents stated that the vaccine will be beneficial, effective, and important for their child’s health and the health of others (Figure 2). More than two-thirds strongly or somewhat agreed that they will do what their child’s doctor or health care provider recommends about the COVID-19 vaccine. On the other hand, many parents agreed that a COVID-19 vaccine might cause lasting health problems for their child or were concerned about serious vaccine side-effects and the novelty of the vaccine.
Figure 2.
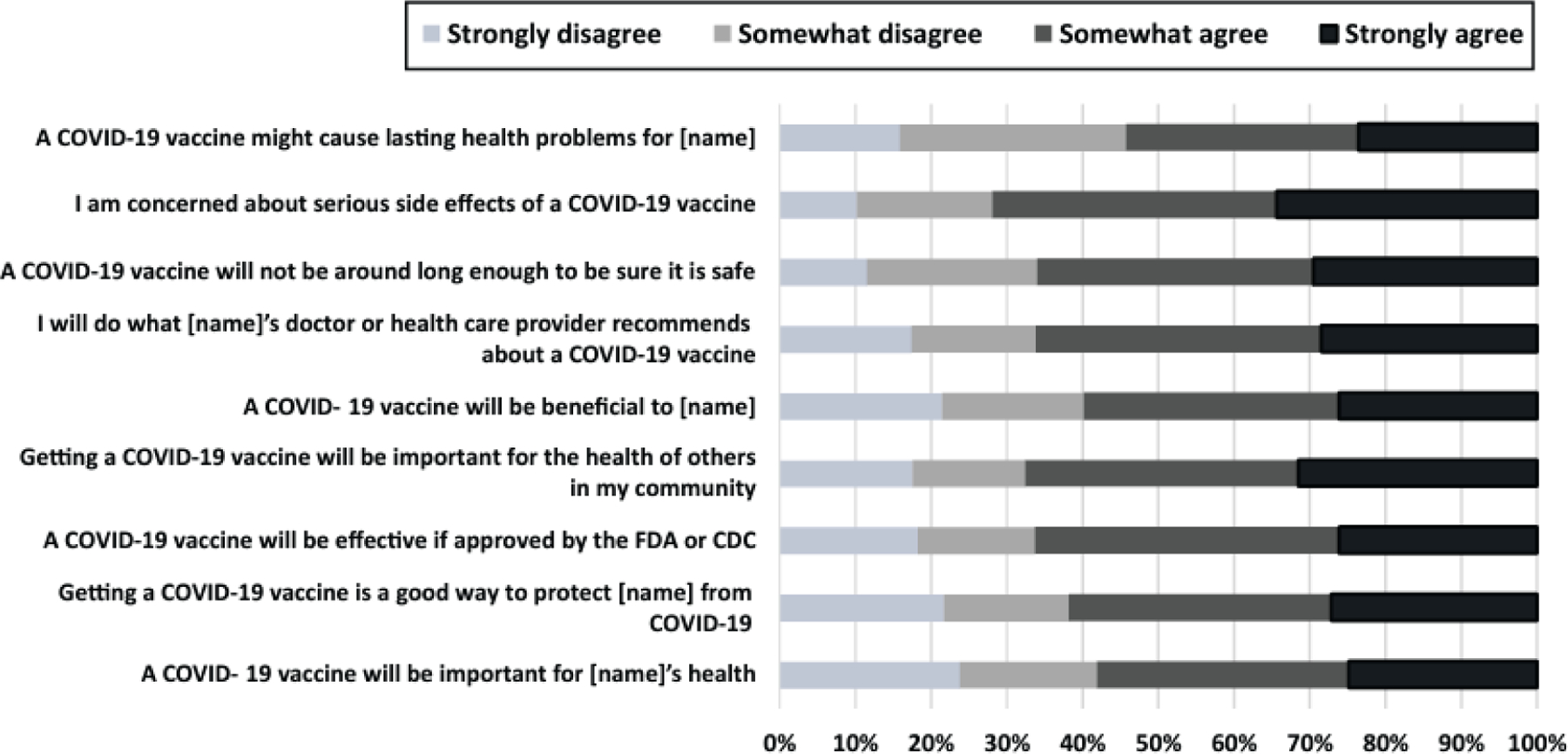
Parental perceptions about COVID-19 vaccination for their child from the 9-item modified Vaccine Hesitancy Scale (VHS), adapted to COVID-19 vaccination for children.
Table 3 shows parents’ perceptions of COVID-19 vaccines for children, stratified by whether they were “very likely” or “somewhat likely” to get their child vaccinated or not. Parents who were very/somewhat likely to get their child vaccinated were far more likely to agree that COVID-19 vaccines are beneficial and effective, and much less likely to agree that the vaccines are unsafe, than parents who were hesitant about vaccination for their child.
The most trusted source of information about the coronavirus vaccine (Table 4) was the child’s doctor, with 72% of parents stating they completely or mostly trust their child’s doctor. A high proportion of parents also trusted their local public health department, the CDC, and the AAP, as well as the government approval process and the vaccine development process in general. Lower levels of trust were observed for information from schools, coworkers, classmates, or other acquaintances, and lowest regarding information from social media.
Table 4.
Parents’ level of trust in sources of information.
| How much do you trust the following sources of information about the coronavirus vaccine?* | |||||
|---|---|---|---|---|---|
| Trust Completely | Trust Mostly | Trust Somewhat | Do Not Trust | Not Applicable | |
| Sources of information about coronavirus vaccine | |||||
| Child’s doctor | 35.7% | 36.1% | 21.3% | 4.4% | 2.5% |
| Child’s school or school district | 9.9% | 23.4% | 35.6% | 17.6% | 13.6% |
| Your local public health department | 11.4% | 31.4% | 40.9% | 15.4% | 0.8% |
| The CDC | 21.1% | 29.0% | 29.5% | 19.8% | 0.7% |
| The American Academy of Pediatrics (AAP) | 18.6% | 29.1% | 35.0% | 15.1% | 2.3% |
| Your close friends and members of your family | 5.8% | 24.0% | 50.7% | 18.4% | 1.1% |
| Your coworkers, classmates, other acquaintances | 2.2% | 14.4% | 48.6% | 32.5% | 2.3% |
| Social media (e.g. Facebook, Instagram, Twitter) | 0.9% | 3.7% | 33.7% | 59.9% | 1.9% |
| Vaccine approval or development process | |||||
| Government approval process for COVID-19 vaccine for child | 9.3% | 30.0% | 28.3% | 32.4% | - |
| Vaccine development process in general for child | 12.8% | 34.1% | 29.0% | 24.1% | - |
The parent-child pair was the unit of analysis. Parents’ sampling weights were used in the analyses to account for design effects.
As Table 5 shows, trust in the child’s doctor, school/school district, the local public health department, the CDC, the AAP, and the vaccine approval and development process were all highly associated with parents stating that they were likely to get a COVID-19 vaccine for their child.
Table 5.
Percent of parents who are very or somewhat likely to get a vaccine for their children, by trust in information sources, the governmental approval process, and vaccine development
| Percent of parents who are very or somewhat likely to vaccinate their children against COVID-19* | |||||
|---|---|---|---|---|---|
| Trust Completely | Trust Mostly | Trust Somewhat | Do Not Trust | P value | |
| Sources of information about coronavirus vaccine | |||||
| Child’s doctor | 71.3% | 46.0% | 14.1% | 14.5% | P<0.0001 |
| Child’s school or school district | 67.8% | 67.9% | 43.4% | 19.2% | P<0.0001 |
| Your local public health department | 88.1% | 66.0% | 34.4% | 9.3% | P<0.0001 |
| The CDC* | 79.7% | 62.3% | 30.8% | 11.8% | P<0.0001 |
| The American Academy of Pediatrics (AAP) | 82.6% | 62.8% | 29.6% | 10.4% | P<0.0001 |
| Your close friends and members of your family | 45.0% | 54.4% | 44.6% | 41.6% | P=0.0828 |
| Your coworkers, classmates, other acquaintances | 54.3% | 50.4% | 47.7% | 42.0% | P=0.0900 |
| Social media (e.g. Facebook, Instagram, Twitter) | 49.1% | 66.5% | 48.2% | 43.9% | P=0.0361 |
| Vaccine approval or development process | |||||
| Government approval process for COVID-19 vaccine for child | 90.7% | 79.4% | 40.1% | 8.3% | P<0.0001 |
| Vaccine development process in general for child | 86.2% | 69.1% | 32.9% | 8.8% | P<0.0001 |
The parent-child pair was the unit of analysis. Parents’ sampling weights were used in the analyses to account for design effects.
CDC, Centers for Disease Control & Prevention
Table 6 presents results of the multivariate analyses, demonstrating the independent association of parent characteristics, child characteristics, and parental trust in information sources with parents stating that they are “very likely” or “somewhat likely” to have their child get the COVID-19 vaccine. Results are shown for four models. Model 1 includes parent and child demographic characteristics and parents’ political affiliation. Older parents and those with higher education are more likely to state their child will receive a vaccine, while Black parents were less likely. Model 2 adds the questions on trust; parental characteristics are no longer independently associated with likelihood of the child getting a COVID-19 vaccine but trust in the child’s doctor or social media and the vaccine development/approval process are associated. Model 3 adds whether the child received an influenza vaccination in the prior two years; this factor is associated with likelihood of future COVID-19 vaccination (aRR=1.44, 95% CI 1.24–1.67). Model 4 adds whether parents received or are likely to receive a COVID-19 vaccine themselves; this factor is the strongest predictor (aRR=3.42, 95% CI 2.32,5.04) while other predictors are the child’s prior influenza vaccination, and trust in the child’s doctor, social media, and the government approval process for COVID-19 vaccines. The child’s age was not associated with likelihood of vaccination in any of the adjusted models.
Table 6.
Multivariate analysis of likelihood of child COVID-19 vaccination by parent and child characteristics*
| Model 1 Parent factors, child factors |
Model 2 Parent factors, child factors and parent perceptions |
Model 3 Adds whether child received influenza vaccination |
Model 4 Adds parents’ COVID-19 vaccination status or likelihood of vaccination |
||
|---|---|---|---|---|---|
| Percent Very Likely or Likely to Get a Vaccine for the Child | Adjusted RR (95% CI) | Adjusted RR (95% CI) | Adjusted RR (95% CI) | Adjusted RR (95% CI) | |
| Parent Characteristics | |||||
| Overall | 46.3% | ||||
| Parental Age (years) | |||||
| 18–39 | 41.1% | - REF - | - REF - | - REF - | - REF - |
| 40–49 | 54.5% | 1.08 (0.90, 1.30) | 1.07 (0.93, 1.24) | 1.09 (0.95, 1.26) | 1.06 (0.93, 1.21) |
| 50+ | 55.4% | 1.25 (1.00, 1.55) | 1.14 (0.97, 1.36) | 1.15 (0.97, 1.36) | 1.04 (0.89, 1.21) |
| Parental Gender | |||||
| Female | 41.7% | - REF - | - REF - | - REF - | - REF - |
| Male | 52.7% | 1.27 (1.08, 1.49) | 1.11 (0.99, 1.26) | 1.16 (1.03, 1.30) | 1.11 (0.99, 1.24) |
| Parental Education | |||||
| High School or Less | 38.5% | - REF - | - REF - | - REF - | - REF - |
| Some College | 40.0% | 1.01 (0.80, 1.29) | 0.89 (0.73, 1.07) | 0.86 (0.71, 1.03) | 0.81 (0.69, 0.97) |
| Bachelor’s or More | 59.0% | 1.36 (1.10, 1.70) | 0.95 (0.81, 1.12) | 0.89 (0.76, 1.05) | 0.84 (0.72, 0.99) |
| Parental Race/Ethnicity | |||||
| White | 45.5% | - REF - | - REF - | - REF - | - REF - |
| Hispanic | 51.8% | 0.90 (0.72, 1.12) | 0.91 (0.76, 1.08) | 0.93 (0.78, 1.11) | 0.94 (0.79, 1.13) |
| Black | 33.8% | 0.50 (0.36, 0.70) | 0.99 (0.77, 1.28) | 1.03 (0.80, 1.33) | 0.97 (0.78, 1.21) |
| Asian | 63.1% | 0.97 (0.75, 1.24) | 1.00 (0.79, 1.26) | 0.98 (0.77, 1.24) | 0.93 (0.74, 1.15) |
| Other | 48.1% | 0.99 (0.64, 1.53) | 0.90 (0.62, 1.33) | 0.88 (0.63, 1.23) | 0.95 (0.67, 1.34) |
| Parental Political Affiliation | |||||
| Democrat | 61.4% | - REF - | - REF - | - REF - | - REF - |
| Republican | 33.6% | 0.97 (0.75, 1.24) | 0.74 (0.64, 0.87) | 0.77 (0.67, 0.90) | 0.81 (0.71, 0.93) |
| Other | 40.9% | 0.99 (0.64, 1.53) | 0.90 (0.76, 1.05) | 0.91 (0.77, 1.07) | 0.92 (0.79, 1.07) |
| Parent received or likely to receive COVID vaccine | 75.2% | 3.42 (2.32, 5.04) | |||
| Child Characteristics | |||||
| Child’s Age (years) | |||||
| 11–18 | 50.7% | - REF - | - REF - | - REF - | - REF - |
| 5–10 | 45.9% | 0.96 (0.84, 1.10) | 0.99 (0.89, 1.11) | 0.98 (0.88, 1.10) | 1.00 (0.90, 1.12) |
| <5 years | 39.3% | 0.84 (0.69, 1.02) | 0.94 (0.79, 1.13) | 0.97 (0.82, 1.15) | 0.94 (0.79, 1.12) |
| Child received influenza vaccine (prior 2 years) | 66.1% | 1.44 (1.24, 1.67) | 1.28 (1.11, 1.48) | ||
| Trust in sources or processes ** | |||||
| Child’s doctor | 58.6% | 1.79 (1.28, 2.48) | 1.62 (1.17, 2.25) | 1.40 (1.04, 1.88) | |
| Child’s school or school district | 67.8% | 1.04 (0.92, 1.18) | 1.06 (0.94, 1.20) | 1.05 (0.93, 1.18) | |
| Local public health department | 71.9% | 1.08 (0.88, 1.32) | 1.08 (0.89, 1.32) | 1.06 (0.88, 1.28) | |
| The CDC | 69.7% | 1.30 (0.93, 1.81) | 1.30 (0.93, 1.81) | 1.14 (0.83, 1.56) | |
| The AAP | 70.5% | 1.13 (0.84, 1.54) | 1.12 (0.82, 1.52) | 1.12 (0.83, 1.52) | |
| Close friends and family members | 52.6% | 0.88 (0.78, 1.01) | 0.87 (0.76, 0.98) | 0.92 (0.80, 1.05) | |
| Coworkers, classmates, acquaintances | 51.0% | 0.94 (0.76, 1.16) | 0.94 (0.76, 1.16) | 0.92 (0.75, 1.14) | |
| Social media | 63.1% | 1.42 (1.11, 1.83) | 1.51 (1.17, 1.96) | 1.47 (1.10, 1.95) | |
| Government approval process for COVID-19 vaccine | 82.1% | 1.76 (1.39, 2.24) | 1.72 (1.38, 2.16) | 1.30 (1.03, 1.63) | |
| Vaccine development process in general | 73.8% | 1.46 (1.12, 1.91) | 1.41 (1.10, 1.81) | 1.24 (0.97, 1.58) |
The parent-child pair was the unit of analysis. Parents’ sampling weights were used in the analyses to account for design effects.
493 respondents indicated “not applicable” for one or more of the trust items, and were excluded from the regression analysis.
Among parents who stated that they “trust completely” or “trust mostly” these sources or processes (versus parents who stated that they “trust somewhat,” or “do not trust.”
CDC, Centers for Disease Control & Prevention); AAP, American Academy of Pediatrics.
Discussion
We found a high level of parental hesitancy for COVID-19 vaccines for children. Less than half of parents state that they are very or somewhat likely to have their children get a COVID-19 vaccine; one-third are very unlikely, and many state that they will “wait and see.” Parents who are white or Black were less likely than those who are Latino or Asian, although these disparities disappeared when we controlled for other parent and child factors. Many parents are concerned about vaccine safety and side-effects, although most also feel that the vaccines will be effective in protecting their children and others. The most important trusted source of information about COVID-19 vaccines for children is their children’s doctor. However, less than half of parents stated that they trust their local health department, the CDC and the AAP, or the vaccine approval and development process. Political affiliation was strongly related to parent-stated intention to vaccinate their child: parents with Democratic affiliation were far more likely to get their child vaccinated. Finally, parents’ own COVID-19 vaccination or likelihood of vaccination was the most important factor independently associated with the likelihood of the child getting a vaccine, although the child’s prior influenza vaccination as well as trust in the child’s doctor, social media, and the vaccine approval process were also independently associated with likelihood of getting the child vaccinated.
Despite high levels of vaccine hesitancy, an encouraging finding is that many parents want to “wait and see,” and may become more interested in the vaccines as results of the pediatric trials are disseminated and more parents and older children are vaccinated. This might mirror the trend seen for adult COVID-19 vaccination, with interest in the vaccines rising over time, at least among older adults and Black individuals.31,32 Notably, many parents are hesitant about COVID-19 vaccination for themselves, which is highly predictive of their hesitancy about the vaccines for their children. Strong outreach is needed to address vaccine hesitancy for both parents and children, including targeted educational efforts to parents who want to “wait and see” about the vaccines for children.
Since vaccine safety and side-effects were key parental concerns, clear messages and transparent communications from public health, government, and leaders about vaccine safety for children are critical. Parental skepticism about the vaccine development and approval processes points to the need for continued transparency and active public education regarding the rigorous development and approval process by the FDA, ACIP and CDC.33,34 The current vaccine safety monitoring system for COVID-19 vaccines is robust,35 and it is important to emphasize the safety profile of the vaccines for children from both the vaccine trials and from post-approval data that will accumulate rapidly as children are vaccinated.
A major finding is that the most trusted source of information about COVID-19 vaccines is the child’s doctor or healthcare provider. Prior studies show that recommendations and effective communication by primary care clinicians have a large impact on vaccine receipt.11,36,37 It is important for pediatric providers to communicate about COVID-19 vaccines for children during routine office visits, even before the vaccines are approved for younger children. Once vaccines are approved, providers can reach out to patients using reminder/recall communications,38,39 information placed on practice websites, and in-office educational information for parents. Our findings on trust and political affiliation highlight a role for specific trusted sources/messengers including political leaders who might be effective in reassuring parents who remain hesitant about the vaccines.
Prior studies and published documents highlight approaches to address parental hesitancy for vaccines in general11,37,40–42 which should also be considered for COVID-19 vaccines.43 Some useful techniques42 might include: using motivational interviewing communication (e.g., asking parents’ permission to discuss COVID-19 vaccines for their child44); telling stories45 (e.g., the practice’s experience with children who were sick from SARS-CoV-2 infection); relating positive experiences regarding the healthcare provider’s own children or other children in the practice receiving COVID-19 vaccines (i.e., “narrative medicine”46); reminding parents why children need COVID-19 vaccines (e.g., making it safer to participate in sports and group activities and to attend school); and reinforcing social norms47 (e.g., stating that “many of our practice’s children are getting the vaccine”).
Our study has strengths and limitations. We surveyed a large, nationally representative sample of parents using an online panel with a high response rate. One limitation involves generalizability from any sample, although the UAS sampling and recruitment approach mitigated these concerns. Our age groups do not align perfectly with current vaccine approval of above 12 years. We had small numbers of Asian respondents. Some respondents might have completed prior UAS surveys32 about adult COVID-19 vaccination, although that should not bias their responses about their children’s vaccination. We do not know what specific factors about the vaccine approval and development processes drove parents’ mistrust. Further, the UAS was designed to sample American adults and not children; thus, our findings reflect the general population of parents although our analyses are at the child level and percentages reflect percent of children. Finally, this survey was conducted in March 2021, and the public’s perception of COVID-19 vaccines can change rapidly, particularly as vaccines are recommended for younger children or if rare side-effects are noted.48,49
In summary, our nationally representative survey found high rates of parental hesitancy for COVID-19 vaccines, with many parents unlikely or unsure they will get their child vaccinated or wanting to “wait and see” about the vaccines. Since pediatric providers were noted to be the most trusted sources of information about COVID-19 vaccines in children, the pediatric community has a major role in promoting and vaccinating the nation’s children against SARS-CoV-2.
What’s Known on This Subject:
Very little is known about parental hesitancy for COVID-19 vaccines for children.
What This Study Adds:
This nationally representative survey found that less than one-half of US parents are likely to have their child receive the COVID-19 vaccine when it is available.
Role of Funder/Sponsor:
The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflict of Interest Disclosures:
The authors have no conflicts of interest to report. This work was supported by the UCLA David Geffen School of Medicine - Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research Award Program; the University of Southern California; the Bill & Melinda Gates Foundation, Seattle, WA; and by Federal funds from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through the Clinical and Translational Science Awards (CTSA) Program (grant number UL1TR001881), the National Institute on Aging (grant number 5U01AG054580-03), and the National Science Foundation (grant number 2028683).
Abbreviations:
- COVID-19
Coronavirus disease 2019
- MIS-C
Multisystem inflammatory syndrome in children
- ACIP
Advisory Committee on Immunization Practices
- CDC
Centers for Disease Control
- VHS
Vaccine Hesitancy Scale
- AAP
American Academy of Pediatrics
- UAS
Understanding America Study
- HPV
Human Papilloma Virus
- FDA
Food and Drug Administration
References
- 1.American Academy of Pediatrics (AAP). Children and COVID-19: State-level data report. 2021. Available at: https://services.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-state-level-data-report/. Accessed May 5, 2021.
- 2.Patrick SW, Henkhaus LE, Zickafoose JS, et al. Well-being of Parents and Children During the COVID-19 Pandemic: A National Survey. Pediatrics. 2020;146(4). [DOI] [PubMed] [Google Scholar]
- 3.Van Dyke ME, Mendoza MCB, Li W, et al. Racial and Ethnic Disparities in COVID-19 Incidence by Age, Sex, and Period Among Persons Aged <25 Years - 16 U.S. Jurisdictions, January 1-December 31, 2020. MMWR Morb Mortal Wkly Rep. 2021;70(11):382–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orskov S, Frost Nielsen B, Fons S, Sneppen K, Simonsen L. The COVID-19 pandemic: Key considerations for the epidemic and its control. APMIS. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oliver SE, Marin M, Wallace M, et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Moderna COVID-19 Vaccine — United States, December 2020. . MMWR Morb Mortal Wkly Rep 2020:1922–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Pfizer-BioNTech COVID-19 Vaccine - United States, December 2020. MMWR Morb Mortal Wkly Rep. 2020;69(50):1922–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oliver SE, Gargano JW, Scobie H, et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Janssen COVID-19 Vaccine - United States, February 2021. MMWR Morb Mortal Wkly Rep. 2021;70(9):329–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wallace M, Woodworth KR, Gargano JW, et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Pfizer-BioNTech COVID-19 Vaccine in Adolescents Aged 12–15 Years - United States, May 2021. MMWR Morb Mortal Wkly Rep. 2021;70(20):749–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Couzin-Frankel J Vaccine trials ramp up in children and adolescents. Science. 2021;371(6532):874–875. [DOI] [PubMed] [Google Scholar]
- 10.Thompson LA, Rasmussen SA. Children and COVID-19 Vaccines. JAMA Pediatr. 2021. [DOI] [PubMed] [Google Scholar]
- 11.Edwards KM, Hackell JM. Countering Vaccine Hesitancy. Pediatrics. 2016;138(3). [DOI] [PubMed] [Google Scholar]
- 12.Dube E, Ward JK, Verger P, MacDonald NE. Vaccine Hesitancy, Acceptance, and Anti-Vaccination: Trends and Future Prospects for Public Health. Annu Rev Public Health. 2021;42:175–191. [DOI] [PubMed] [Google Scholar]
- 13.Kempe A, Saville AW, Albertin C, et al. Parental Hesitancy About Routine Childhood and Influenza Vaccinations: A National Survey. Pediatrics. 2020;146(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szilagyi PG, Albertin CS, Gurfinkel D, et al. Prevalence and characteristics of HPV vaccine hesitancy among parents of adolescents across the US. Vaccine. 2020;38(38):6027–6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson E, Jones A, Lesser I, Daly M. International estimates of intended uptake and refusal of COVID-19 vaccines: A rapid systematic review and meta-analysis of large nationally representative samples. Vaccine. 2021;39(15):2024–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szilagyi PG, Thomas K, Shah MD, et al. National Trends in the US Public’s Likelihood of Getting a COVID-19 Vaccine-April 1 to December 8, 2020. Jama. 2020;325(4):396–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen KH, Srivastav A, Razzaghi H, et al. COVID-19 Vaccination Intent, Perceptions, and Reasons for Not Vaccinating Among Groups Prioritized for Early Vaccination - United States, September and December 2020. MMWR Morb Mortal Wkly Rep. 2021;70(6):217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisher KA, Bloomstone SJ, Walder J, Crawford S, Fouayzi H, Mazor KM. Attitudes Toward a Potential SARS-CoV-2 Vaccine : A Survey of U.S. Adults. Ann Intern Med. 2020;173(12):964–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romer D, Jamieson KH. Conspiracy theories as barriers to controlling the spread of COVID-19 in the U.S. Soc Sci Med. 2020;263:113356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daly M, Robinson E. Willingness to Vaccinate Against COVID-19 in the U.S.: Representative Longitudinal Evidence From April to October 2020. Am J Prev Med. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rhodes ME, Sundstrom B, Ritter E, McKeever BW, McKeever R. Preparing for A COVID-19 Vaccine: A Mixed Methods Study of Vaccine Hesitant Parents. J Health Commun. 2020;25(10):831–837. [DOI] [PubMed] [Google Scholar]
- 22.Skjefte M, Ngirbabul M, Akeju O, et al. COVID-19 vaccine acceptance among pregnant women and mothers of young children: results of a survey in 16 countries. Eur J Epidemiol. 2021;36(2):197–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montalti M, Rallo F, Guaraldi F, et al. Would Parents Get Their Children Vaccinated Against SARS-CoV-2? Rate and Predictors of Vaccine Hesitancy According to a Survey over 5000 Families from Bologna, Italy. Vaccines (Basel). 2021;9(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelly BJ, Southwell BG, McCormack LA, et al. Correction to: Predictors of willingness to get a COVID-19 vaccine in the U.S. BMC Infect Dis. 2021;21(1):383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly BJ, Southwell BG, McCormack LA, et al. Predictors of willingness to get a COVID-19 vaccine in the U.S. BMC Infect Dis. 2021;21(1):338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kapteyn A, Angrisani M, Bennett D, et al. Tracking the Effect of the COVID-19 Pandemic on American Households. Survey Research Methods. 2020;14(2):179–186. [Google Scholar]
- 27.World Health Organization, Vaccines and Biologicals. SAGE working group dealing with vaccine hesitancy (March 2012 to November 2014). Available at: https://www.who.int/immunization/sage/sage_wg_vaccine_hesitancy_apr12/en/. Accessed May 5, 2021.
- 28.Shapiro GK, Tatar O, Dube E, et al. The vaccine hesitancy scale: Psychometric properties and validation. Vaccine. 2018;36(5):660–667. [DOI] [PubMed] [Google Scholar]
- 29.Luyten J, Bruyneel L, van Hoek AJ. Assessing vaccine hesitancy in the UK population using a generalized vaccine hesitancy survey instrument. Vaccine. 2019;37(18):2494–2501. [DOI] [PubMed] [Google Scholar]
- 30.Akel KB, Masters NB, Shih SF, Lu Y, Wagner AL. Modification of a vaccine hesitancy scale for use in adult vaccinations in the United States and China. Hum Vaccin Immunother. 2021:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szilagyi PG, Thomas K, Shah MD, et al. Likelihood of COVID-19 Vaccination By Subgroups Across the US: Post-Election Trends and Disparities. Human Vaccines & Immunotherapeutics (In Press). 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daly M, Jones A, Robinson E, An increase in willingness to vaccinate against COVID-19 in the US between October 2020 and February 2021: longitudinal evidence from the Understanding America Study. medRxiv 2021.03.04.21252918; doi: 10.1101/2021.03.04.21252918. [DOI] [Google Scholar]
- 33.Bell BP, Romero JR, Lee GM. Scientific and Ethical Principles Underlying Recommendations From the Advisory Committee on Immunization Practices for COVID-19 Vaccination Implementation. JAMA. 2020;324(20):2025–2026. [DOI] [PubMed] [Google Scholar]
- 34.Lee GM, Bell BP, Romero JR. The Advisory Committee on Immunization Practices and Its Role in the Pandemic Vaccine Response. JAMA. 2020;324(6):546–547. [DOI] [PubMed] [Google Scholar]
- 35.Shimabukuro TT. COVID-19 vaccine safety update. 2021.
- 36.Dempsey AF, O’Leary ST. Human Papillomavirus Vaccination: Narrative Review of Studies on How Providers’ Vaccine Communication Affects Attitudes and Uptake. Acad Pediatr. 2018;18(2S):S23–S27. [DOI] [PubMed] [Google Scholar]
- 37.Braun C, O’Leary ST. Recent advances in addressing vaccine hesitancy. Curr Opin Pediatr. 2020;32(4):601–609. [DOI] [PubMed] [Google Scholar]
- 38.Szilagyi PG, Albertin CS, Saville AW, et al. Effect of State Immunization Information System Based Reminder/Recall for Influenza Vaccinations: A Randomized Trial of Autodialer, Text, and Mailed Messages. J Pediatr. 2020;221:123–131 e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacobson Vann JC, Jacobson RM, Coyne-Beasley T, Asafu-Adjei JK, Szilagyi PG. Patient reminder and recall interventions to improve immunization rates. Cochrane Database Syst Rev. 2018;1:CD003941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brewer NT, Chapman GB, Rothman AJ, Leask J, Kempe A. Increasing Vaccination: Putting Psychological Science Into Action. Psychol Sci Public Interest. 2017;18(3):149–207. [DOI] [PubMed] [Google Scholar]
- 41.Jarrett C, Wilson R, O’Leary M, Eckersberger E, Larson HJ, Hesitancy SWGoV. Strategies for addressing vaccine hesitancy - A systematic review. Vaccine. 2015;33(34):4180–4190. [DOI] [PubMed] [Google Scholar]
- 42.UNICEF. Vaccine Messaging Guide. December 2020. Available at: https://www.unicef.org/media/93661/file/Vaccine%20messaging%20guide.pdf. Accessed May 5, 2021.
- 43.Coyne-Beasley T, Hill SV, Zimet G, et al. COVID-19 Vaccination of Adolescents and Young Adults of Color: Viewing Acceptance and Uptake With a Health Equity Lens. J Adolesc Health. 2021;68(5):844–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reno JE, O’Leary S, Garrett K, et al. Improving Provider Communication about HPV Vaccines for Vaccine-Hesitant Parents Through the Use of Motivational Interviewing. Journal of health communication. 2018;23(4):313–320. [DOI] [PubMed] [Google Scholar]
- 45.Shelby A, Ernst K. Story and science: how providers and parents can utilize storytelling to combat anti-vaccine misinformation. Hum Vaccin Immunother. 2013;9(8):1795–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Charon R At the membranes of care: stories in narrative medicine. Acad Med. 2012;87(3):342–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goldstein S, MacDonald NE, Guirguis S, Hesitancy SWGoV. Health communication and vaccine hesitancy. Vaccine. 2015;33(34):4212–4214. [DOI] [PubMed] [Google Scholar]
- 48.Marshall M, Ferguson ID, Lewis P, et al. Symptomatic Acute Myocarditis in Seven Adolescents Following Pfizer-BioNTech COVID-19 Vaccination. Pediatrics. 2021. [DOI] [PubMed] [Google Scholar]
- 49.O’Leary ST, Maldonado YA. Myocarditis after SARS-CoV-2 Vaccination: True, True, and…Related? Pediatrics. 2021. [DOI] [PubMed] [Google Scholar]


