Abstract
Background
Treating facial aging with CO2 lasering or microneedling are cornerstones of facial rejuvenation. Skin rejuvenation utilizing thermal and mechanical treatments have historically been considered too injurious to be combined at a single setting. Autologous nanofat has been shown to deliver wound healing properties. We investigated the safety and efficacy of co-terminus CO2 lasering and microneedling to resolve fine lines and rhytids in facial skin with addition of autologous nanofat to aid in recovery.
Objectives
Combination treatments may result in better results with faster recovery. We investigated the safety and efficacy of co-terminus CO2 lasering and microneedling to resolve fine lines and rhytids in facial skin with addition of autologous nanofat to aid in recovery.
Methods
Twenty-three patients underwent facial treatment with CO2 lasering followed by microneedling and application of autologous nanofat (LaMiNa). One volunteer patient had tissue biopsies of treatment areas to demonstrate histologic tissue level changes.
Results
All patients verbally reported no pain (Numerical Rating System 0–10) following procedure and had rapid recovery within an average of 5 days. Pathology results demonstrated that CO2 and microneedling had persistent epidermal disruption and perineural inflammation at 4 days, while the introduction of autologous nanofat at the time of CO2 and microneedling resulted in full recovery of epidermis and resolution of perineural inflammation.
Conclusions
Triple therapy (LaMiNa) with thermal CO2 remodeling and mechanical microneedling penetration have accelerated and pain-free recovery with the addition of autologous nanofat. Histologic analysis reveals that epidermal recovery is accelerated and perineural inflammation is reduced with the addition of autologous nanofat following skin remodeling from combined CO2 and microneedling.
Level of Evidence: 3

Ablative carbon-dioxide (CO2) lasering, minimally invasive percutaneous collagen induction (microneedling), and autogenous nanofat injection are 3 minimally invasive techniques that can address a wide array of dermatologic issues including skin rejuvenation, scarring, and rhytids.1-3 Historically, ablative CO2 lasering has been used as a stand-alone technique that targets water by emitting 10,600 nm light pulse that leads to vaporization of both intracellular and extracellular water, resulting in tissue ablation and destruction. The depth of dermal ablation and surrounding tissue destruction depend on the duration and intensity of exposure to the laser.4 The destruction and the depth of tissue injury seen with CO2 lasering can lead to persistent erythema and prolonged recovery times. These adverse qualities have caused CO2 lasering to be used with caution as a stand-alone technique, rarely combining it with other skin rejuvenation modalities.
Microneedling is a mechanical process which percutaneously punctures the skin down to the reticular dermis. Fibroblasts stimulated within the dermis are then induced to produce collagen. This process is frequently paired with the delivery of collagen, platelet-rich plasma, or autologous nanofat.5-9
Autologous nanofat is a liquid derivative of fatty tissue obtained by destroying adipocytes through an emulsification and straining process. It consists of a condensation of stromal vascular fraction (SVF) along with intracellular hormones and cytokines. Because of these unique components, among others, it has been shown to have regenerative properties.10-15
The combination treatment of CO2 lasering, microneedling, and autologous nanofat is a novel procedure that we present as a proof of concept demonstrating the additive benefits of thermal and mechanical tissue manipulation followed by a “rescue.” We refer to this minimally invasive treatment as LaMiNa: laser, microneedling, and nanofat. Introduction of autologous nanofat into the reticular dermis through microneedling, immediately following lasering, may be a key adjunct for the resolution of degenerative skin lesions, where pain is minimized and recovery is accelerated.
METHODS
The study was designed to demonstrate that the combination of 3 mainstay treatments of facial rejuvenation is feasible and is without adverse events. IRB Exemption was granted by Institutional Review Board E-22-5193 CO2 Laser, Microneedling and Nanofat for Facial Rejuvenation: A Retrospective Review on July 7, 2022. Written consent was provided where the patients agreed to the use of their analysis of their data.
All patients were treated by the senior author under local anesthesia with topical anesthetic benzocaine 20%, lidocaine 10%, and tetracaine10% (Sincerus, Pompano Beach, FL) with supplemental infraorbital and mental nerve blocks with 1% lidocaine. The CO2 laser treatment using Tetra SmartXide CO2 (Cartessa Aesthetics, Melville, NY) was delivered in 2 applications. The first application was performed in DP setting (25 watts, 1000 μs dwell time, 500 μm spacing, for 13% coverage). This was performed in the area with the deepest rhytids along the upper and lower lip. The subsequent application was in HP setting (3.5 watts, 0 spacing, for 78% coverage) covering the same areas. After both CO2 applications, the patients immediately underwent microneedling using SkinPen (Crown Aesthetics, Dallas, TX) apparatus with depth set to 2.5 mm, with an endpoint of pinpoint bleeding. Nanofat was then harvested from autologous lipoaspirate, processed through sequentially smaller connectors, and separated through a Nanofilter (Tulip Medical, San Diego, CA) to achieve a maximum diameter of 500 microns.1 Nanofat was then applied to the previously treated skin and the microneedling procedure repeated to allow penetration through the epidermis. Repeat applications of nanofat were applied after the prior aliquot had been absorbed into the tissue for a total of 20 cc of autologous nanofat.
Patient demographics, skin characteristics, procedural details, and outcomes were recorded. All patients were provided with a pain scale score card (0-10) immediately after the procedure, as well as during follow-up visits and/or phone calls on posttreatment Days 3 and 6 and verbally responses were recorded. Full recovery was further defined as patients being comfortable to socialize at an intimate distance without a mask based on subjective commentary.
One volunteer provided an opportunity for control histopathologic analysis. The volunteer (control) was a male age 54, with Fitzpatrick Type 1 skin and no pertinent medical history. The medial aspect of the right upper arm and left upper arm were marked with 3 areas measuring 1 cm × 1 cm. These areas were labeled as 1, 2, 3 on the left arm and 4, 5, 6 on the right arm. Prior to treatment, the entire area was anesthetized with 1% lidocaine. Treatments inside the sites were as follows: Site 1: control, no treatment; Site 2: microneedling with nanofat; Site 3: CO2 laser resurfacing, microneedling, and nanofat (LaMiNa treatment); Site 4: CO2 laser resurfacing with nanofat; Site 5: CO2 laser resurfacing, and microneedling alone; Site 6: CO2 laser resurfacing, microneedling, and nanofat (LaMiNa treatment). Treatment Sites 1, 2, and 3 underwent immediate posttreatment punch biopsy and Treatment Sites 4, 5, and 6 were biopsied at 4 days posttreatment. All specimens were sent for hematoxylin and eosin (H&E) stain.
RESULTS
Twenty-three patients, all female, with desire for elimination of perioral rhytids volunteered to participate in the study from August 2020 to June 2022. The mean patient age was 60.7 years (range 32-82), and mean BMI was 23.9 (range 19.1-33.8). These patients either had a prior facelift surgery or declined alternative invasive treatments to manage their perioral rhytids. One patient with a history of perioral herpes simplex was given prophylactic antiviral medication prior to the procedure.
All patients reported a 0 pain score (on a numeric rating scale: of 0-10) immediately after the procedure, as well as on Days 3 and 6. These were determined by review of postoperative records and clinic note records during which patients were queried about pain levels. A full recovery was reported on average by 5.3 days. No patients experienced a herpetic outbreak, and no adverse events were evidenced.
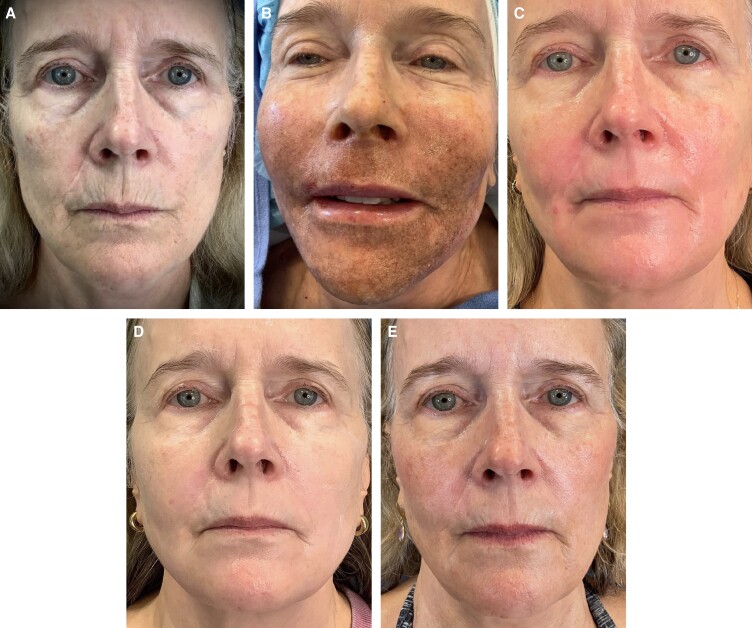
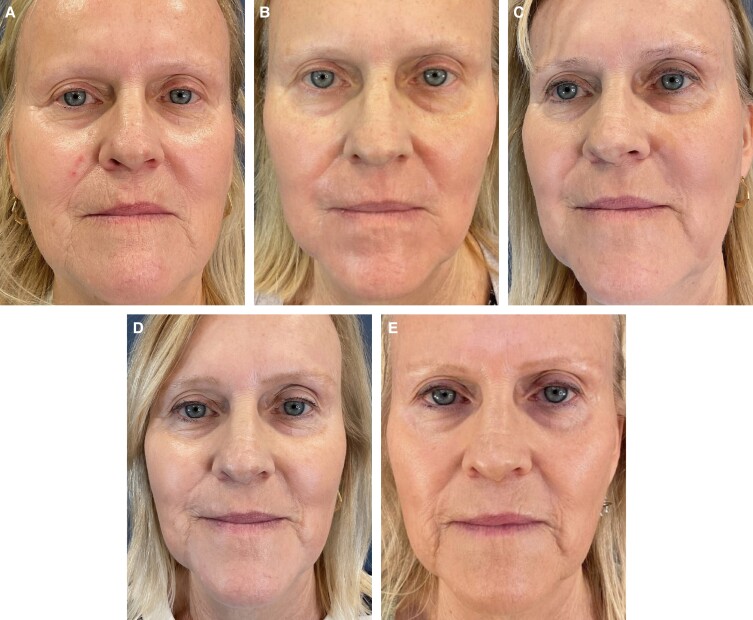
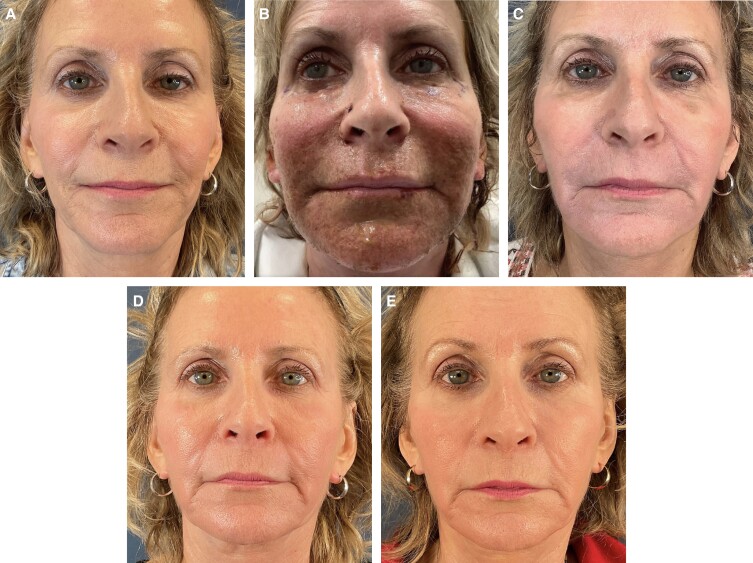
Patient photographs demonstrate before and after treatment illustrating significant improvement in fine lines and rhytids along her perioral region. Patient 1 is a 63-year-old female. The effect of the treatment is demonstrated in her immediate posttreatment images. Her early hyperemia is resolved completely by 2 weeks and the results are sustained at the 6-month follow-up photographs (Figure 1). Patient 2 is a 67-year-old female. Her photographs show pretreatment perioral rhytids which are resolved at 2 weeks with results that are sustained at her 2-month, 4-month, and 1-year follow-up evaluations (Figure 2). Patient 3 is a 67-year-old female. Her photographs show pretreatment perioral rhytids and Day 1 postprocedure treatment and her results at 2 weeks, 3 months, and 6-month follow-up (Figure 3).
Figure 1.
A 63-year-old female patient shown (A) preoperative, (B) immediately postoperative, (C) 2 weeks posttreatment, (D) 2 months posttreatment, and (E) 6 months posttreatment.
Figure 2.
A 67-year-old female patient shown (A) preoperative, (B) 2 weeks posttreatment, (C) 2 months posttreatment, (D) 4 months posttreatment, and (E) 12 months posttreatment.
Figure 3.
A 67-year-old female shown (A) preoperative, (B) 1 day posttreatment, (C) 2 weeks posttreatment, (D) 3 months posttreatment, and (E) 6 months posttreatment.
An adult male volunteer underwent treatment of the upper inner arm. The areas were either biopsied immediately or 4 days postprocedure. Figure 4 represents tissue biopsied as the control with no treatment. Figure 5 shows the area that was treated with microneedling and autologous nanofat and immediate biopsy after treatment. Figure 6 shows the area that was treated with CO2 laser, microneedling, and autologous nanofat, which is the LaMiNa treatment and immediate biopsy following treatment. Figure 7 shows the area that was treated with just CO2 laser resurfacing and then biopsied at postprocedure Day 4. Figure 8 shows the area that was treated with CO2 laser resurfacing, and microneedling alone. This area was biopsied at postprocedure Day 4. Figure 9 shows the area that was treated with LaMiNa treatment (CO2 laser, microneedling, and autologous nanofat) and then biopsied at postprocedure Day 4. Figure 10 is a higher magnification of Figure 9: LaMiNa treatment at postprocedure Day 4 demonstrating increased level of cellular turnover as evidenced by cells captured in metaphase of mitosis with the chromosomes condensing in the midline of the nucleus as they prepare to divide.
Figure 4.
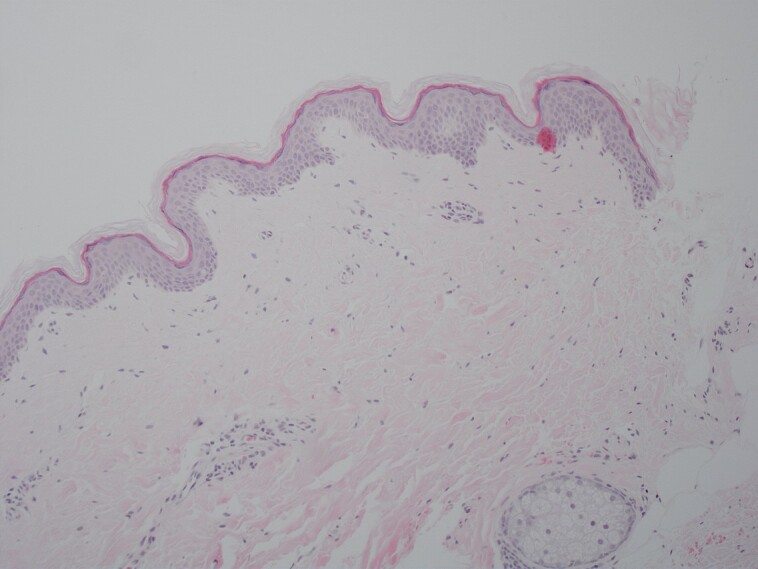
Representation of tissue biopsied as control with no treatment.
Figure 5.
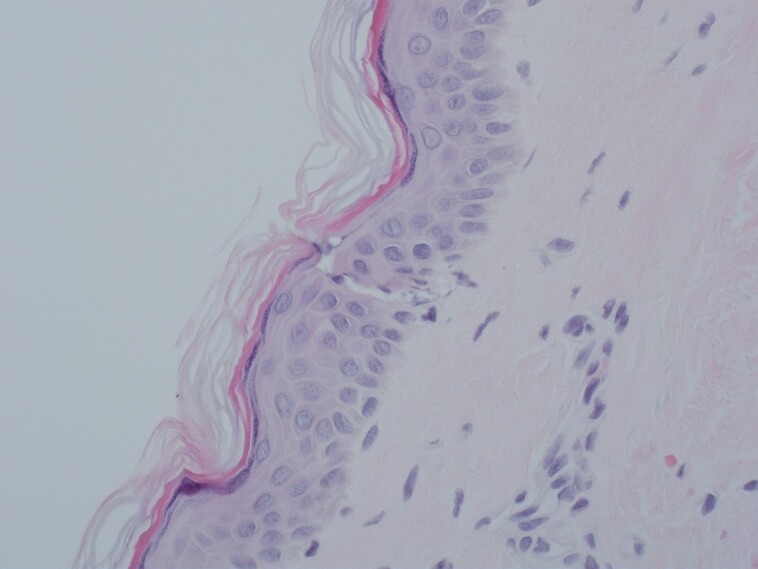
The area shown underwent microneedling and autologous nanofat and immediate biopsy after treatment.
Figure 6.
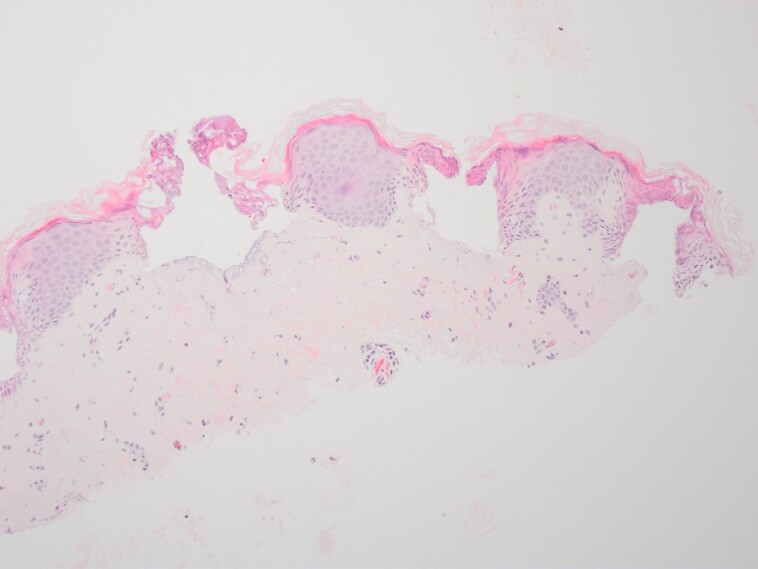
The area shown underwent CO2 laser, microneedling, and autologous nanofat, which is the LaMiNa treatment and immediate biopsy following treatment.
Figure 7.
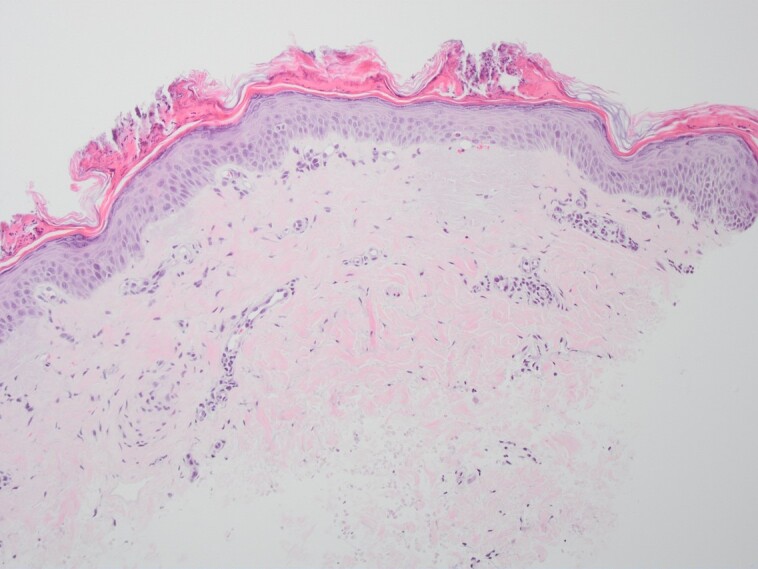
The area shown was treated with just CO2 laser resurfacing and then biopsied at postprocedure Day 4.
Figure 8.
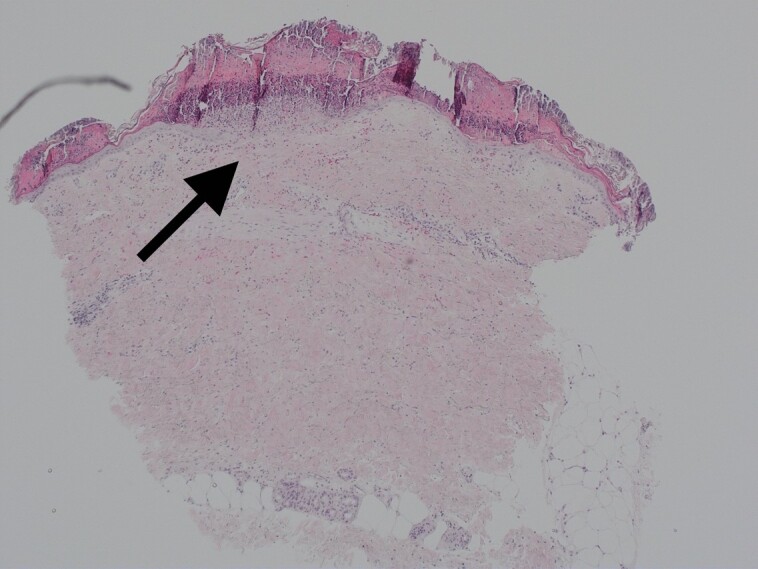
The area shown was treated with CO2 laser resurfacing and microneedling alone. This area was biopsied at postprocedure Day 4.
Figure 9.
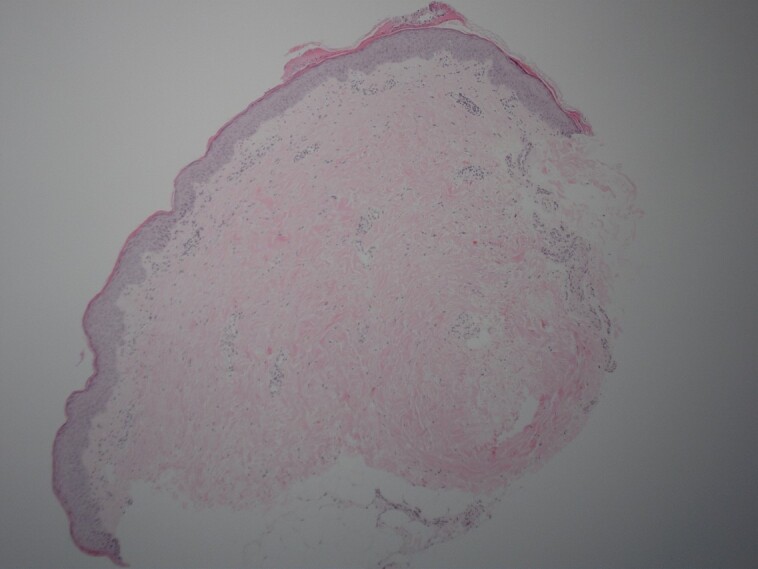
The area was treated with LaMiNa treatment (CO2 laser, microneedling, and autologous nanofat) and then biopsied at postprocedure Day 4.
Figure 10.
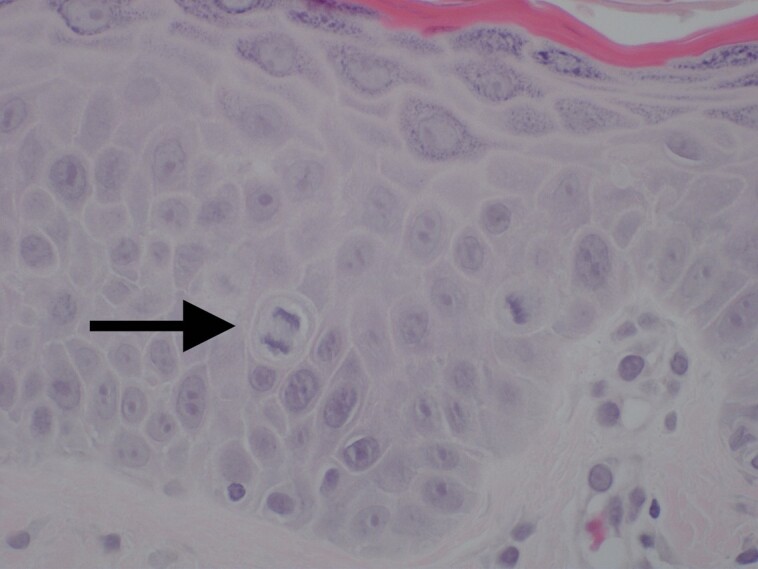
A higher magnification of Figure 9: LaMiNa treatment at postprocedure Day 4 demonstrating increased level of cellular turnover as evidenced by cells captured in metaphase of mitosis with the chromosomes condensing in the midline of the nucleus as they prepare to divide.
Biopsy specimens focusing on the perineural tissue show baseline levels in Figure 11 with minimal inflammation. Figure 12 shows a significant amount of perineural inflammation with extravasated neutrophils and monocytes when tissue is treated with only CO2 laser and microneedling, as indicated by the arrow. This is in contrast with the tissue treated with LaMiNa that included CO2 laser with microneedling and nanofat application. The result as seen in Figure 13 was a significant reduction in perineural inflammation, as indicated by the arrow.
Figure 11.
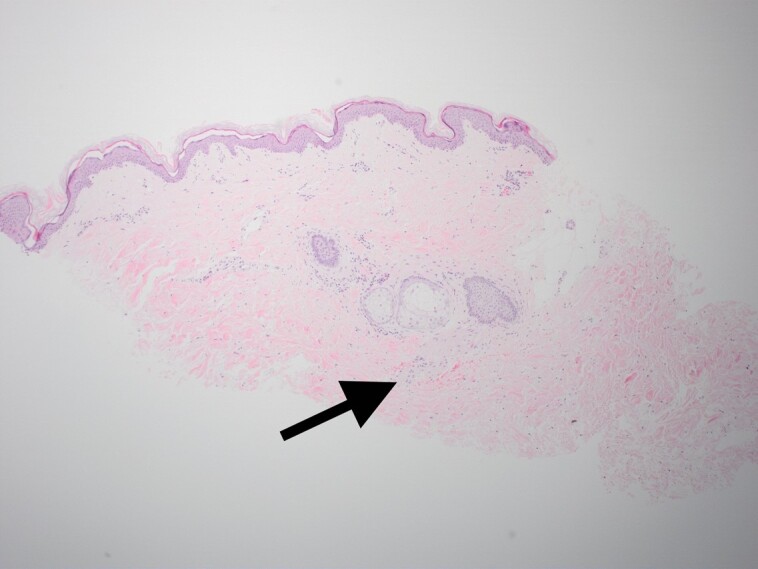
Biopsy specimens focusing on the perineural tissue show baseline levels with minimal inflammation.
Figure 12.
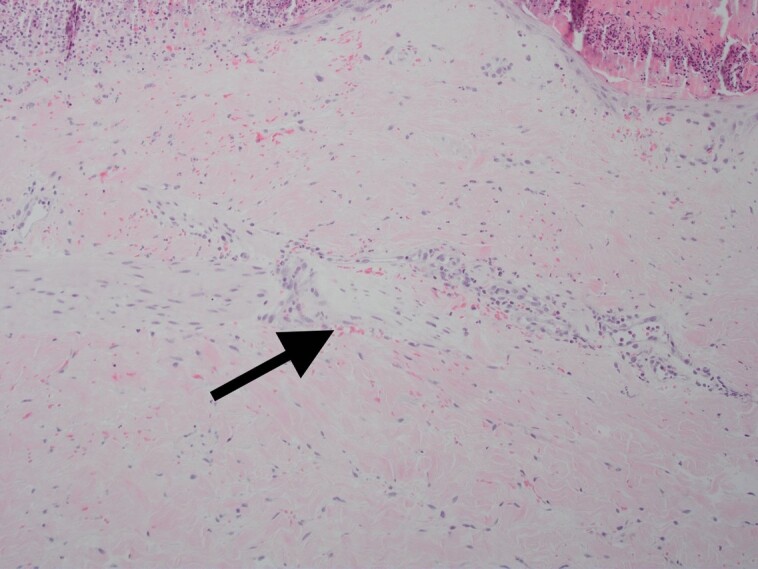
A significant amount of perineural inflammation with extravasated neutrophils and monocytes is shown when tissue is treated with only CO2 laser and microneedling (arrow). This is in contrast with the tissue treated with LaMiNa that included CO2 laser with microneedling and nanofat application.
Figure 13.
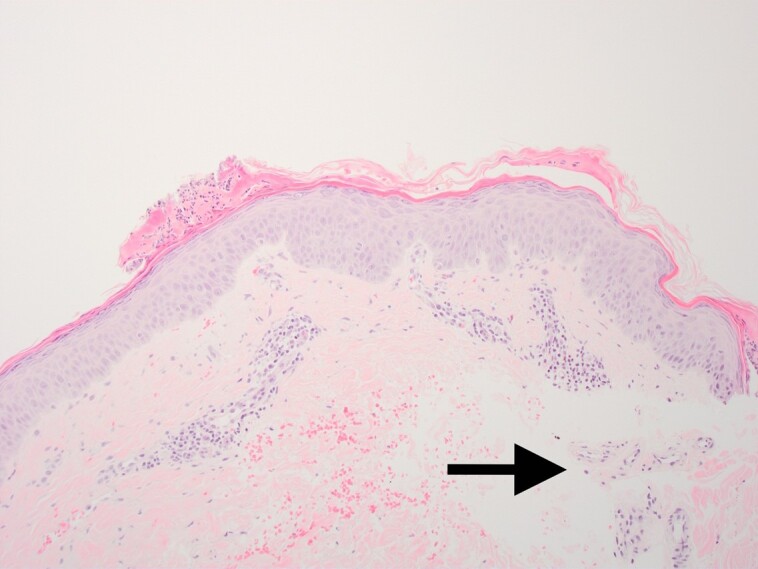
The result was a significant reduction in perineural inflammation (arrow).
DISCUSSION
As the aging process advances, the epidermis thins, glycosaminoglycans, collagen, and elastin are diminished at the dermal layer, and fine lines and wrinkles begin to form. As facial rejuvenation techniques continue to evolve, the modern discerning patient is seeking minimally invasive methods to deliver a youthful appearance with rapid recovery and minimal pain.
Historically, microneedling or lasering has been used as a mainstay for facial rejuvenation through mechanical or thermal tissue modulation, respectively. These methods stimulate inflammation and collagen production. The more robust the treatment, the more prolonged the adverse side effects, such as hyperemia and pain both resulting in a delay in the resumption of baseline social activity.
It is acknowledged that skin differs between body parts and gender. Our use of skin from the medial aspect of the upper arm in a male patient is an initial attempt to better understand the physiologic responses to our novel treatment algorithm. Future studies are planned where histologic samples will be from the actual areas of facial treatment in the female patients. The single (control) patient tissue biopsies did however allow for a basic understanding of human tissue biologic response to the combination of thermal trauma from vaporization of water containing cells by the CO2 laser treatment followed immediately by mechanical trauma of microneedling inducing a skin injury pattern of high magnitude and the contribution of autologous nanofat to accelerated recovery. The treatment resulted in significant extra-capillary extravasation of red blood cells, monocytes, polymorphonuclear neutrophils, and likely other mediators of the innate immune system throughout the dermis and reticular dermis. The epidermal layer remained interrupted with interspersed fibrin and serum forming a crust over the dermis. This can be seen in Figure 8 biopsies (CO2 lasering and microneedling at POD4). Historically, practitioners have avoided such an aggressive (combined) treatment regimen due to the associated pain and prolonged recovery time. It was exactly this concern that prompted the addition of autologous nanofat to the treatment algorithm as a “rescue” adjuvant: rescue from both pain and prolonged recovery time.
The molecular mechanisms for the procedural pain associated with CO2 lasering and microneedling are complex. Nociceptors are primary afferent neurons which transmit noxious stimuli to the brain. These neurons can be activated by both thermal and mechanical injury. It was found that pain from exposure to elevated temperatures, as experienced with the CO2 laser is generated by activation of the transient receptor potential (TRP) family of nociceptors.16 In the case of CO2 laser treatments, thermal injury above 43° centigrade activates the TRP nociceptors which use medium (A-delta) to small diameter (C-fibers) axons to transmit the signal of pain to the central nervous system. The deep thermal injury from CO2 lasering induces an injury pattern which often results in painful response which can last for days due to the thermally injured nerve endings in the reticular and dermal layers.17 On the other hand, the patient experience during and after a microneedling session is mostly a pain-free experience with the exception of mild discomfort experienced during the actual treatment.
Adult adipose tissue contains progenitor cells. These progenitor cells are termed adipose-derived stem cells (ASCs) and have the capacity for multilineage differentiation.18 SVF is a component of lipoaspirate collected during the preparation of nanofat and contains a large population of the ASCs, as well as other mesenchymal progenitor cells, leukocyte subtypes, pericytes, and vascular smooth muscle cells. SVF is a reservoir of cells with regenerative and healing capabilities; they have paracrine actions that may stimulate angiogenesis, modulate inflammation, and act as antioxidants.19-21
Autologous nanofat may therefore contain mediators which ameliorate nociceptor pain response through key neuronal kinases present in the milieu of nanofat. It has been recently hypothesized that these kinases may modulate nociceptor firing through phosphorylating pain transducing ion channels thereby reducing perceived pain.22,23
When autologous nanofat was added to our treatment protocol, the resultant clinical experience for the patient was virtually pain free. This is reported universally and immediately in our patient cohort with 0/10 pain scores at posttreatment Days 0, 3, and 6 during postprocedure evaluation and questioning. This is in contrast with the results of Batra et al who performed a prospective study of patient experiences after laser resurfacing, and patients reported discomfort for a mean of 10.9 days postoperatively.24 The pain blocking effect of the nanofat seems not only to have an immediate effect but also a sustained effect with virtually no pain experienced throughout the recovery process. This may indicate that the benefits of nonfat application may extend beyond the temporary humoral (innate immune response) benefits and possibly include cellular elements (adaptive immune response) activated as a result of ruptured adipose cells. It is quite possible that intact SVF or mesenchymal stem cells also accelerate the recovery process and repair the injured nerve endings.25,26
During the microneedling procedure, tens of thousands of microchannels are created extending down 2.5 mm through the papillary dermis into the reticular dermis. These channels are roughly 1000 microns in diameter and create a temporary path for the 500 microns size autologous nanofat to transit the dermal layer down to the reticular dermis. It has been previously reported that microneedling combined with absorption of nanofat induces fibroblasts and stimulates the release of platelets, releasing cytokines and growth factors to stimulate collagen production.27
Hypererythema seen following CO2 lasering monotherapy can last for ∼5 weeks.4 In our study, patients reported a full recovery, defined as being able to comfortably leave the house without makeup or facial covering on or before 5 days. The rapid healing and recovery are not only clinically evident but also evident in the tissue biopsies where inflammation has all but dissipated in our experimental (triple therapy) group (Figure 6).
Specifically, when comparing Figure 6, which shows the immediate damage caused by the LaMiNa treatment, to Figure 9, which shows the healing after only 4 days with LaMiNa treatment, the immediate destruction of the epidermal layer and deeper injury to the dermis are replaced by a thin and compacted stratum corneum with an intact layer of epidermal cells and minimal evidence of monocytes, polymorphonuclear neutrophils infiltration. In Figure 10, under a higher magnification of the epidermal layer, an increased level of cellular turnover can be seen as evidenced by cells captured in metaphase of mitosis with the chromosomes condensing in the midline of the nucleus as they prepare to divide. However, after 4 days when no “rescue” autologous nanofat is utilized, there is histologically significant inflammation characterized by extra-capillary extravasation of red blood cells, monocytes, polymorphonuclear neutrophils, and other mediators of the innate immune system throughout the dermis and reticular dermis (Figure 8). The epidermal layer remained interrupted with interspersed fibrin and serum forming a crust over the dermis. The histological evidence demonstrates increased perineural inflammation in the CO2 laser and microneedling alone in Figure 12 while the control (Figure 11) and the LaMiNa treatment (Figure 13) show minimal perineural inflammation.
Possible explanation for these finding may be that monocytes and neutrophils, activated by the thrombin released from the microneedling can phosphorylate Heat Shock Protein 27 which is known to enhance endothelial barrier permeability.28 This activated pathway, among others, may explain the findings of perineural inflammation in Figure 12 and explain why the addition of autologous nanofat mitigates the innate immune response and diminishes the perineural inflammatory cascade in Figure 13.
Furthermore, it is well documented that thermal and mechanical induced skin trauma, as seen with CO2 laser and microneedling, lead to localized damage-associated molecular patterns (DAMPs). These injury patterns induce increased endothelial permeability and subsequent increased leukocyte extravasation.29 High-Mobility Group Protein Box 1 (HMGPB1), which is the most studied nuclear peptide of DAMPs, can be either passively released into the extracellular matrix by necrotic factors from dead or dying cells or HMGPB1 can be signaled for release by inflammatory mediators. When found in the extracellular milieu, HMGPB1 activates the nonspecific innate immune system through rapid cellular translocation due to its small 25,000 Dalton size. HMGPB1 is a potent neuroinflammatory mediator.30 Prolonged inflammation interferes with tissue rejuvenation and results in the clinical findings of prolonged and painful recovery. Excessive Inflammation is in conflict with regeneration, and modalities which can more quickly reduce inflammation will accelerate rejuvenation.31
Recently in both the laboratory and clinical arenas, the application of nanofat-containing progenitor cells to damaged skin has revealed encouraging rejuvenating data. In the laboratory, Zhou et al, using a rat model, discovered, after the application of nanofat to burn wounds not only healed faster due to increased rates of cell proliferation but also formed more new micro vessels due to the upregulation of vascular endothelial growth factor production.32 Clinically, Rigotti et al demonstrated the histological effects of ASCs on aged facial skin.18
Although the triple therapy LaMiNa technique is novel, microneedle-based combination therapy is not a totally new concept. Asif et al showed that microneedling monotherapy improved acne scarring but found there was greater improvement with combination microneedling and platelet-rich plasma therapy.5 El-Fakahany et al demonstrated the efficacy of microneedling as a delivery system by showing increased efficacy of topical anesthetic with microneedling prior to application.6 Garg and Baveja suggested the additive benefit of combination treatment of subcision, microneedling, and TCA peel on collagen induction and skin resurfacing.33 Mohammed demonstrated some improvement in ice pick acne scars with CO2 laser pinpoint irradiation and needling.34
In our study, we have presented an additive effect of CO2 lasering, microneedling, and nanofat delivery to the skin as a proof of concept, for safe and accelerated wound recovery with very minimal postprocedure pain. This was demonstrated by the clinical results in the experimental group of 23 female patients and histologically from the arm of a healthy male control subject. While combined CO2 laser and microneedling treatment has not previously been considered a treatment option for facial rejuvenation due to deleterious side effects, we have shown in our series that the “rescue” with autologous nanofat appears to mitigate against these adverse outcomes. Although the benefits of this triple treatment regimen may be multifactorial, one could hypothesize that the effect of reduced inflammation and early rejuvenation may be neurally mediated and that the minimal pain felt by the patient is a signal marker for localized immune response modulation to “communicate” through neural networks signaling for a reduced inflammatory response.
Future studies will address the absence of controls or alternative treatments, such as split face studies to more completely gauge the impact of the LaMiNa treatment. Additionally blinded clinical observers will be employed to measure the rhytid improvement with the aid of the Perioral Rhytids Severity Scale, Global Aesthetic Improvement Scale, and the FACE-Q.35
Limitations of this study include recorded verbal reports subjective measures of pain using the Numerical Rating System 0 to 10 in the medical records. Future studies may employ more complete subjective measurement of pain with the use of McGill Pain Questionnaire or the Pain Rating Index system in addition to the use of the Numerical Rating System.36
CONCLUSIONS
Our study has limitations based on the small number of patients treated and the fact that the comparable tissue biopsy histology from only male patient's arm skin may not accurately correlate with the female facial skin. We acknowledge that the study design does not include a control (for ethical reasons); however, we did trial one patient with platelet-rich plasma in place of autologous nanofat and evidenced an increase in postoperative pain as described by the patient with less dramatic resolution of fine lines and wrinkles. Clinical observations of our patient's results demonstrate improved fine lines, wrinkles, and rhytids with a pain-free rapid recovery following the application of combined CO2 lasering, microneedling, and autologous nanofat treatment (LaMiNa). Tissue biopsy results from a male patient's inner arm treated with LaMiNa correlate with clinical findings with a faster epithelial layer recovery and less perineural and dermal inflammation compared to CO2 and microneedling without autologous nanofat.
This report outlines that combined thermal and mechanical skin resurfacing can be safely performed in conjunction with a “rescue” element delivered through autologous nanofat, and that histological results have demonstrated that the dermal inflammation seen following CO2 lasering combined with microneedling is mitigated by the application of nanofat. The mechanism may be mediated by neural communication at the local skin level. Further study is certainly warranted to elucidate the exact mechanism of action and use of outcomes tools such as the FACE-Q.
Disclosures
Dr Claytor is a stockholder and investor in Love My Delta (Philadelphia, PA), and a stockholder and research for Alastin (Dallas, TX). The remaining authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
The authors received no financial support for the research, authorship, and publication of this article, including payment of the article processing charge.
REFERENCES
- 1. Tonnard P, Verpaele A, Peeters G, Hamdi M, Cornelissen M, Declercq H. Nanofat grafting: basic research and clinical applications. Plast Reconstr Surg. 2013;132(4):1017–1026. doi: 10.1097/PRS.0b013e31829fe1b0 [DOI] [PubMed] [Google Scholar]
- 2. Liebl H, Kloth LC. Skin cell proliferation stimulated by microneedles. J Am Coll Clin Wound Spec. 2012;4(1):2–6. doi: 10.1016/j.jccw.2012.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hamilton M, Campbell A, Holcomb JD. Contemporary laser and light-based rejuvenation techniques. Facial Plast Surg Clin North Am. 2018;26(2):113–121. doi: 10.1016/j.fsc.2017.12.002 [DOI] [PubMed] [Google Scholar]
- 4. Neaman KC, Baca ME, Piazza RC, VanderWoude DL, Renucci JD. Outcomes of fractional CO2 laser application in aesthetic surgery: a retrospective review. Aesthet Surg J. 2010;30(6):845–852. doi: 10.1177/1090820X10386930 [DOI] [PubMed] [Google Scholar]
- 5. Asif M, Kanodia S, Singh K. Combined autologous platelet-rich plasma with microneedling verses microneedling with distilled water in the treatment of atrophic acne scars: a concurrent split-face study. J Cosmet Dermatol. 2016;15(4):434–443. doi: 10.1111/jocd.12207 [DOI] [PubMed] [Google Scholar]
- 6. El-Fakahany H, Medhat W, Abdallah F, Abdel-Raouf H, Abdelhakeem M. Fractional microneedling: a novel method for enhancement of topical anesthesia before skin aesthetic procedures. Dermatol Surg. 2016;42(1):50–55. doi: 10.1097/DSS.0000000000000580 [DOI] [PubMed] [Google Scholar]
- 7. Quinlan DJ, Ghanem AM, Hassan H. Topical growth factors and home-based microneedling for facial skin rejuvenation. J Cosmet Dermatol. 2022;21(8):3469–3478. doi: 10.1111/jocd.14650 [DOI] [PubMed] [Google Scholar]
- 8. Tonnard P, Verpaele A, Carvas M. Fat grafting for facial rejuvenation with nanofat grafts. Clin Plast Surg. 2020;47(1):53–62. doi: 10.1016/j.cps.2019.08.006 [DOI] [PubMed] [Google Scholar]
- 9. Righesso R, Piccinini PS, Uebel CO. A combined approach for fast nanofat microneedling. J Cutan Aesthet Surg. 2021;14(2):248–255. doi: 10.4103/JCAS.JCAS_142_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liang ZJ, Lu X, Li DQ, et al. . Precise intradermal injection of nanofat-derived stromal cells combined with platelet-rich fibrin improves the efficacy of facial skin rejuvenation. Cell Physiol Biochem. 2018;47(1):316–329. doi: 10.1159/000489809 [DOI] [PubMed] [Google Scholar]
- 11. Suh A, Pham A, Cress MJ, et al. . Adipose-derived cellular and cell-derived regenerative therapies in dermatology and aesthetic rejuvenation. Ageing Res Rev. 2019;54:100933. doi: 10.1016/j.arr.2019.100933 [DOI] [PubMed] [Google Scholar]
- 12. Xiao S, Zhang F, Zheng Y, et al. . Synergistic effect of nanofat and mouse nerve-growth factor for promotion of sensory recovery in anterolateral thigh free flaps. Stem Cells Transl Med. 2021;10(2):181–189. doi: 10.1002/sctm.20-0226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lombardo JA, Banyard DA, Widgerow AD, Haun JB. Fluidic device system for mechanical processing and filtering of human lipoaspirate enhances recovery of mesenchymal stem cells. Plast Reconstr Surg. 2023;151(1):72e–84e. doi: 10.1097/PRS.0000000000009798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schilling BK, Baker JS, Komatsu C, et al. . Intramuscular nanofat injection promotes inflammation-induced gastrocnemius regeneration in syngeneic rat sciatic nerve injury model. Plast Reconstr Surg. 2022. doi: 10.1097/PRS.0000000000010115[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 15. Verpaele A, Tonnard P, Jeganathan C, Ramaut L. Nanofat needling: a novel method for uniform delivery of adipose-derived stromal vascular fraction into the skin. Plast Reconstr Surg. 2019;143(4):1062–1065. doi: 10.1097/PRS.0000000000005455 [DOI] [PubMed] [Google Scholar]
- 16. Kamkin A, Kiseleva I, eds. Mechanosensitivity in Cells and Tissues. Academia; 2005. [PubMed] [Google Scholar]
- 17. Nwonu CNS. Neuronal cell mechanisms of pain. West Afr J Med. 2022;39(10):1075–1983. [PubMed] [Google Scholar]
- 18. Rigotti G, Charles-de-Sá L, Gontijo-de-Amorim NF, et al. . Expanded stem cells, stromal-vascular fraction, and platelet-rich plasma enriched fat: comparing results of different facial rejuvenation approaches in a clinical trial. Aesthet Surg J. 2016;36(3):261–270. doi: 10.1093/asj/sjv231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee SH, Jin SY, Song JS, Seo KK, Cho KH. Paracrine effects of adipose-derived stem cells on keratinocytes and dermal fibroblasts. Ann Dermatol. 2012;24(2):136–143. doi: 10.5021/ad.2012.24.2.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rydén M, Dicker A, Götherström C, et al. . Functional characterization of human mesenchymal stem cell-derived adipocytes. Biochem Biophys Res Commun. 2003;311(2):391–397. doi: 10.1016/j.bbrc.2003.10.010 [DOI] [PubMed] [Google Scholar]
- 21. Nguyen A, Guo J, Banyard DA, et al. . Stromal vascular fraction: a regenerative reality? Part 1: current concepts and review of the literature. J Plast Reconstr Aesthet Surg. 2016;69(2):170–179. doi: 10.1016/j.bjps.2015.10.015 [DOI] [PubMed] [Google Scholar]
- 22. Cai R, Chen XZ. Roles of intramolecular interactions in the regulation of TRP channels. Rev Physiol Biochem Pharmacol. 2023:186:29–56 . doi: 10.1007/112_2022_74 [DOI] [PubMed] [Google Scholar]
- 23. Cao Y, Gang X, Sun C, Wang G. Mesenchymal stem cells improve healing of diabetic foot ulcer. J Diabetes Res. 2017;2017:9328347. doi: 10.1155/2017/9328347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Batra RS, Jacob CI, Hobbs L, Arndt KA, Dover JS. A prospective survey of patient experiences after laser skin resurfacing: results from 2 1/2 years of follow-up. Arch Dermatol. 2003;139(10):1295–1299. doi: 10.1001/archderm.139.10.1295 [DOI] [PubMed] [Google Scholar]
- 25. Zhu Z, Zhang X, Hao H, et al. . Exosomes derived from umbilical cord mesenchymal stem cells treat cutaneous nerve damage and promote wound healing. Front Cell Neurosci. 2022;16:913009. doi: 10.3389/fncel.2022.913009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shimizu M, Matsumine H, Osaki H, et al. . Adipose-derived stem cells and the stromal vascular fraction in polyglycolic acid-collagen nerve conduits promote rat facial nerve regeneration. Wound Repair Regen. 2018;26(6):446–455. doi: 10.1111/wrr.12665 [DOI] [PubMed] [Google Scholar]
- 27. Fernandes D. Commentary on: micro-needling depth penetration, presence of pigment particles, and fluorescein-stained platelets: clinical usage for aesthetic concerns. Aesthet Surg J. 2017;37(1):86–88. doi: 10.1093/asj/sjw151 [DOI] [PubMed] [Google Scholar]
- 28. Rada CC, Mejia-Pena H, Grimsey NJ, et al. . Heat shock protein 27 activity is linked to endothelial barrier recovery after proinflammatory GPCR-induced disruption. Sci Signal. 2021;14(698):eabc1044. doi: 10.1126/scisignal.abc1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Relja B, Land WG. Damage-associated molecular patterns in trauma. Eur J Trauma Emerg Surg. 2020;46(4):751–775. doi: 10.1007/s00068-019-01235-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yamada S, Maruyama I. HMGB1, a novel inflammatory cytokine. Clin Chim Acta. 2007;375(1-2):36–42. doi: 10.1016/j.cca.2006.07.019 [DOI] [PubMed] [Google Scholar]
- 31. Ziegler ME, Claytor B, Bell M, Casas L, Widgerow AD. Gene expression changes in the skin of patients undergoing medial thigh liposuction with pre-surgical and post-surgical application of topical products. Aesthet Surg J Open Forum. 2020;2(3):ojaa033. doi: 10.1093/asjof/ojaa033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhou H, Fang Q, Li N, Yu M, Chen H, Guo S. ASMq protects against early burn wound progression in rats by alleviating oxidative stress and secondary mitochondria-associated apoptosis via the Erk/p90RSK/Bad pathway. Mol Med Rep. 2021;23(5):390. doi: 10.3892/mmr.2021.12029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Garg S, Baveja S. Combination therapy in the management of atrophic acne scars. J Cutan Aesthet Surg. 2014;7(1):18–23. doi: 10.4103/0974-2077.129964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mohammed G. Randomized clinical trial of CO2 laser pinpoint irradiation technique with/without needling for ice pick acne scars. J Cosmet Laser Ther. 2013;15(3):177–182. doi: 10.3109/14764172.2013.793584 [DOI] [PubMed] [Google Scholar]
- 35. Sundaram H, Shamban A, Schlessinger J, et al. . Efficacy and safety of a new resilient hyaluronic acid filler in the correction of moderate-to-severe dynamic perioral rhytides: a 52-week prospective, multicenter, controlled, randomized, evaluator-blinded study. Dermatol Surg. 2022;48(1):87–93. doi: 10.1097/DSS.0000000000003238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bendinger T, Plunkett N. Measurement in pain medicine. BJA Education. 2016;16(9):310–315. doi 10.1093/bjaed/mkw014 [DOI] [Google Scholar]