Figure 3.
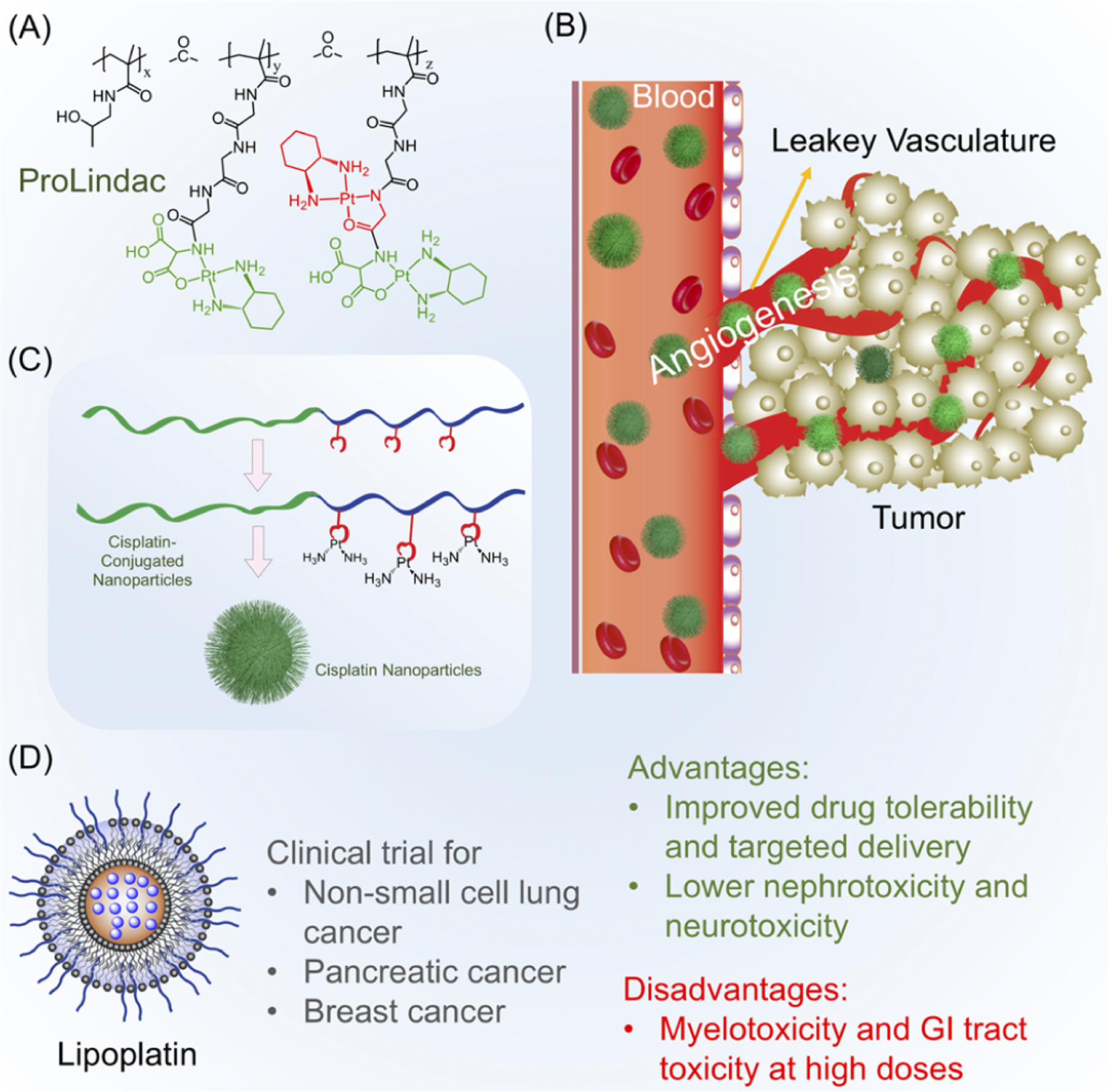
(A) Example of controlled-release polymer-based-drug ProLindac, which allows for the delivery and release of oxaliplatin. (B) Illustration of the enhanced permeability and retention (EPR) effect, which allows for the preferential accumulation of size-optimized NPs at the tumor site. (C) Representation of cisplatin-conjugated polymers and the formation of polymeric NPs for the gradual release of cisplatin. (D) Example of FDA-approved nanomedicine Lipoplatin, which demonstrates improved safety and delivery over those of traditional cisplatin.
