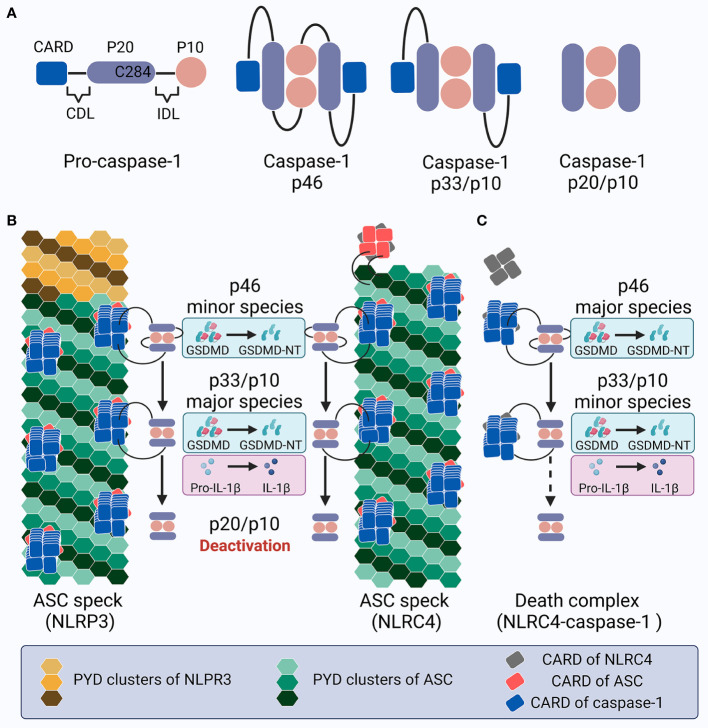
Figure 4.
Schematic showing caspase-1 activation and deactivation. (A) Structure of pro-caspase-1 and caspase-1 species generated by dimerization and auto-processing during inflammasome activation. (B) Upon NLRP3 or NLRC4 inflammasome activation in cells expressing ASC, specks recruit pro-caspase-1 molecules, leading to proximity-induced dimerization, generation of p46 species (minor), and later auto-proteolysis; this process first occurs at the IDL to generate p33/p10 species (major), and subsequently at the CDL to create and dislocate the inactive p20/10 species. The p46 species cleaves GSDMD into GSDMD-NT, while p33/p10 species cleaves both GSDMD and IL-1β. In contrast, the p20/p10 species is incompetent for processing GSDMD and IL-1β. Within the ASC speck, caspase-1 activation and deactivation is rapidly promoted with a high turnover. (C) Upon NLRC4 inflammasome activation in ASC deficient cells, the turnover of p46 into p33/p10, and especially p33/p10 into p20/p10 (illustrated with a dotted arrow), slows down; this leads to prolonged caspase-1 activity, with p46 and p33/p10 as the major and minor species, respectively, which may contribute to the pyroptosis-predominant uncoupling without massive IL-1β release. This inflammasome complex is called the death complex.