Table 3.
BRD4 and CK2 Inhibition Rates and In Vitro Antiproliferative Activity of Compounds 3, 14, 42a–j, 43a–q, and 44a–n
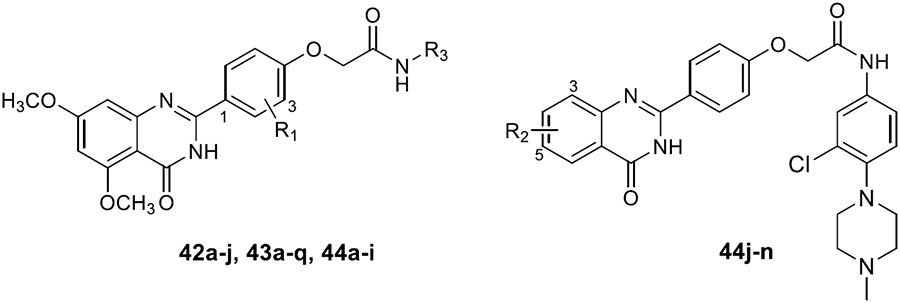
| |||||||
|---|---|---|---|---|---|---|---|
| NO. | R1 | R2 | R3 | Kinase inhibitory activity (1 μM, %)[a] |
Anti-proliferative active (IC50, μM)[b] |
||
| BRIM | CK2 | MDA-MB-231 | MDA-MB-468 | ||||
| 42a | H | - |
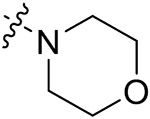
|
45.29 ± 1.42 | 42.20 ± 1.25 | 14.71 ± 1.55 | 12.54 ± 0.87 |
| 42b | 3,5-diCH3 | - |
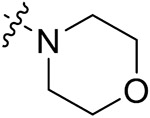
|
42.58 ± 0.82 | 40.31 ± 1.76 | 20.57 ± 0.62 | 18.50 ± 1.23 |
| 42c | H | - |
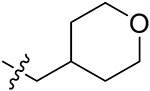
|
43.19 ± 3.47 | 43.43 ± 2.59 | 21.21 ± 2.80 | 26.61 ± 0.85 |
| 42d | 3,5-diCH3 | - |
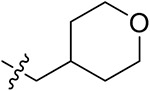
|
37.47 ± 1.42 | 33.70 ± 3.64 | 23.61 ± 0.58 | 25.56 ± 1.57 |
| 42e | 3,5-diBr | - |
|
51.11 ± 3.47 | 47.43 ± 2.19 | 16.93 ± 0.87 | 14.81 ± 1.66 |
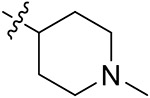
| 42f | 3,5-diCH3 | - |
|
52.38 ± 1.15 | 49.23 ± 2.59 | 17.21 ± 2.80 | 15.55 ± 2.21 |
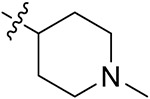
| 42g | H | - |
|
55.71 ± 2.02 | 53.64 ± 1.79 | 12.82 ± 1.72 | 13.65 ± 0.23 |
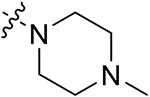
| 42h | H |
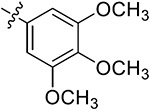
|
54.19 ± 1.28 | 47.43 ± 1.09 | 23.37 ±1.35 | 15.03 ± 0.26 | |
| 42i | 3,5-diCH3 |
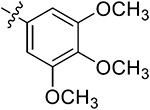
|
53.50 ± 3.47 | 42.14 ± 0.33 | 24.41 ± 2.81 | 16.55 ± 1.80 | |
| 42j | 3,5-diCH3 | - |
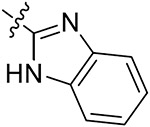
|
56.14 ± 3.09 | 25.61 ± 6.57 | >50 | 30.31 ± 1.56 |
| 43a | H | - |
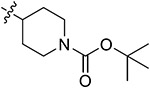
|
62.10 ± 0.52 | 55.68 ± 2.07 | 13.12 ± 1.67 | 16.62 ± 2.21 |
| 43b | 3,5-diCH3 | - |
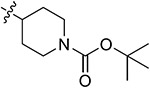
|
57.39 ± 2.06 | 51.38 ± 1.69 | 22.37 ± 2.30 | 17.24 ± 1.63 |
| 43c | 3,5-diCH3 | - |
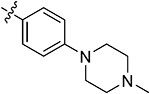
|
78.36 ± 4.07 | 69.31 ± 2.19 | 10.81 ± 1.85 | 12.59 ± 1.24 |
| 43d | 3,5-diCH3 | - |
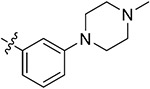
|
72.44 ± 2.87 | 57.08 ± 1.27 | 14.57 ± 1.64 | 15.74 ± 2.66 |
| 43e | 3,5-diBr | - |

|
62.58 ± 0.52 | 58.47 ± 1.23 | 13.64 ± 2.17 | 15.83 ± 1.11 |
| 43f | H | - |
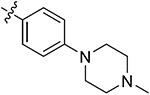
|
82.30 ± 2.73 | 80.02 ± 4.22 | 6.46 ± 0.62 | 8.80 ± 1.15 |
| 43g | H | - |

|
74.33 ± 1.28 | 56.02 ± 1.90 | 13.22 ± 0.54 | 8.07 ± 1.68 |
| 43h | H | - |
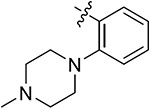
|
69.01 ± 2.30 | 51.44 ± 0.65 | 17.32 ± 0.79 | 11.50 ± 0.54 |
| 43i | 3,5-diCH3 | - |
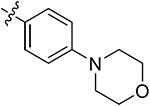
|
81.44 ± 1.15 | 63.61 ± 0.34 | 8.23 ± 1.39 | 9.49 ± 1.18 |
| 43j | 3,5-diCH3 | - |
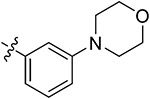
|
73.71 ± 0.83 | 55.73 ± 1.86 | 11.26 ± 0.93 | 13.44 ± 0.77 |
| 43k | 3,5-diBr | - |
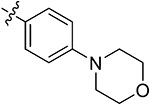
|
67.20 ± 1.52 | 58.29 ± 3.62 | 13.21 ± 2.21 | 15.11 ± 1.75 |
| 43l | H | - |
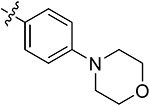
|
80.65 ± 3.98 | 75.39 ± 2.52 | 7.67 ± 1.03 | 9.54 ± 0.74 |
| 43m | H | - |

|
61.74 ± 2.03 | 60.02 ± 1.38 | 15.53 ± 0.91 | 17.80 ± 1.22 |
| 43n | 3,5-diCH3 | - |
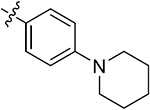
|
69.24 ± 3.67 | 70.51 ± 0.54 | 13.43 ± 2.53 | 9.43 ± 1.09 |
| 43o | 3,5-diBr | - |
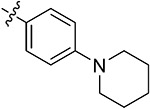
|
56.39 ± 0.46 | 62.47 ± 1.23 | 16.35 ± 2.02 | 15.63 ± 1.26 |
| 43p | H | - |
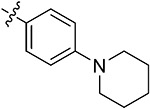
|
71.06 ± 2.96 | 75.20 ± 1.38 | 10.27 ± 2.03 | 11.63 ± 0.46 |
| 43q | H | - |
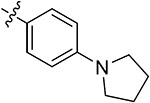
|
68.51 ± 1.72 | 67.52 ± 0.71 | 19.56 ± 1.63 | 7.82 ± 4.02 |
| 44a | 3,5-diCH3 | - |
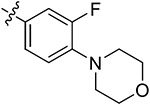
|
83.29 ± 3.45 | 76.21 ± 2.53 | 10.30 ± 1.23 | 9.16 ± 0.93 |
| 44b | H | - |
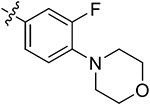
|
85.43 ± 2.65 | 79.61 ± 3.65 | 7.09 ± 0.25 | 8.58 ± 1.51 |
| 44c | H |
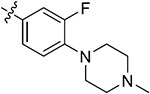
|
85.37 ± 1.58 | 82.46 ± 0.13 | 6.30 ± 2.37 | 7.55 ± 1.73 | |
| 44d | 3,5-diCH3 |
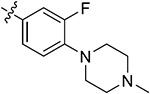
|
87.61 ± 2.73 | 80.23 ± 0.42 | 5.72 ± 1.45 | 8.33 ± 1.17 | |
| 44e | H | - |
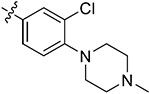
|
92.28 ± 0.66 | 90.65 ± 1.39 | 2.66 ± 0.81 | 3.52 ± 0.58 |
| 44f | 3,5-diCH3 | - |
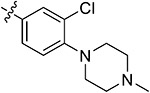
|
89.27 ± 1.47 | 85.40 ± 0.38 | 3.49 ± 1.23 | 4.58 ± 0.52 |
| 44g | 3,5-diBr | - |
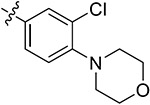
|
79.30 ± 2.50 | 86.29 ± 1.47 | 4.02 ± 1.92 | 5.53 ± 0.28 |
| 44h | H | - |
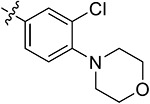
|
83.52 ± 1.54 | 75.19 ± 0.73 | 4.52 ± 0.56 | 8.29 ± 1.92 |
| 44i | 3,5-diCH3 | - |
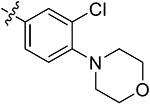
|
85.47 ± 0.28 | 70.13 ± 2.94 | 10.23 ± 0.95 | 6.31 ± 1.94 |
| 44j | - | 4-F | - | 45.26 ± 0.57 | 68.27 ± 1.39 | 6.94 ± 0.68 | 7.51 ± 1.13 |
| 44k | - | 4-OCH3 | - | 55.60 ± 1.62 | 72.03 ± 2.82 | 14.59 ± 0.77 | 16.36 ± 0.85 |
| 44l | - | 4-Cl | - | 51.49 ± 3.02 | 75.61 ± 1.12 | 14.40 ± 0.58 | 15.27 ± 0.72 |
| 44m | - | 5-Cl | - | 47.29 ± 1.16 | 65.93 ± 1.80 | 5.21 ± 0.82 | 6.23 ± 0.55 |
| 44n | - | 5-CH3 | - | 48.20 ± 0.47 | 74.70 ± 2.11 | 17.36 ± 0.56 | 16.22 ± 1.83 |
| 1 | - | - | - | 97.03 ± 1.32 | 6.60 ± 0.41 | 29.51 ± 1.70 | 30.19 ± 0.69 |
| 18 | - | - | - | 7.14 ± 1.51 | 98.53 ± 1.87 | 14.25 ± 0.62 | 20.92 ± 1.04 |
| 1 + | - | - | - | - | - | 7.37 ± 0.39 (CI = 0.78)d | 9.10 ± 0.14 (CI = 0.61d |
| 18 c | |||||||
Reported compounds were tested in triplicate. Data are presented as mean ± SD.
IC50 values were determined from cell viability assay for 24 h.
Combined at a mole ratio of 1:1.
Using the Chou–Talalay method.
