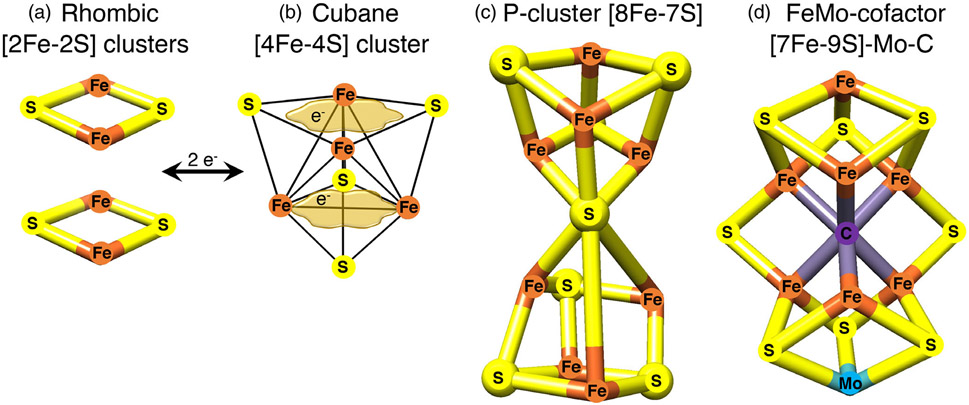
FIGURE 1.
Examples of Fe-S clusters found in proteins. (a) The common rhombic [2Fe-2S] clusters are initially assembled from inorganic iron and sulfur upon the main scaffold ISCU, and they can be utilized to generate much more complex Fe-S cofactors. (b) The tetranuclear or cubane clusters are formed by reductive coupling of two [2Fe-2S] clusters, in a process that requires two electrons (2e−). [4Fe-4S] clusters have the capability to delocalize electrons between iron sites (as depicted by the tan cloud-like frames, which illustrate that the iron-associated electrons are highly delocalized between neighboring iron atoms). (c) Larger Fe-S cofactors can attain much higher complexity in the P-cluster of nitrogenase or in the iron-molybdenum cofactor of nitrogenase depicted in (d), where an interstitial carbon atom occupies the core of the cofactor. Adopted from139