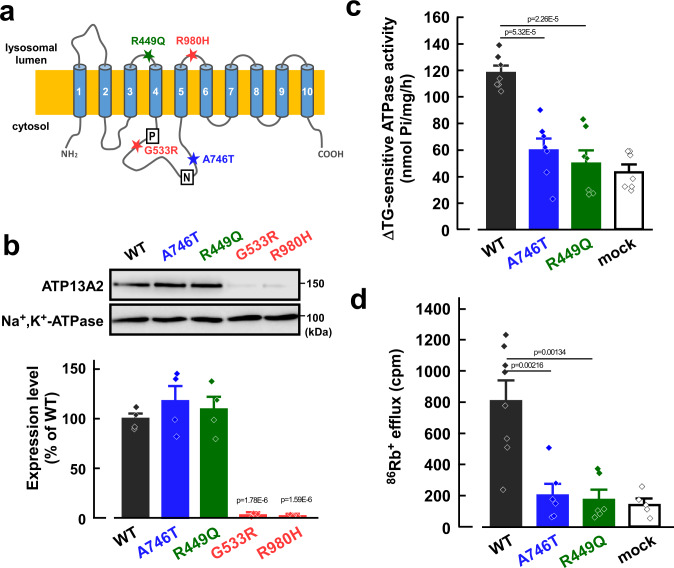
Fig. 2. Effects of PD-associated mutations on ATP13A2-derived activity.
a Topology model of human ATP13A2. PD-associated mutations (R449Q, G533R, A746T, and R980H), phosphorylation domain (P), and nucleotide-binding domain (N) are indicated. b Western blotting for ATP13A2 (~150 kDa) and Na+,K+-ATPase (~100 kDa) in the ATP13A2 WT- and mutants-transfected HEK293 cells. Expression level of ATP13A2 was normalized to that of Na+,K+-ATPase. The expression level of ATP13A2 in WT-transfected cells was taken as 100%. (n = 4 independent replicates). Statistical significance was determined by two-tailed unpaired Student’s t test. c ΔTG-sensitive ATP13A2 activities were measured in the membrane fractions of the ATP13A2 WT-, A746T, and R449Q-transfected HEK293 cells. (n = 7 independent replicates). Statistical significance was determined by two-tailed unpaired Student’s t test. d The 86Rb+ effluxes in the ATP13A2 WT-, A746T- and R449Q-transfected cells. (n = 5–8 independent replicates). Statistical significance was determined by two-tailed unpaired Student’s t test. All data are presented as mean ± SEM. Source data are provided as a source data file.