Figure 2. Circulating Tumor DNA (ctDNA) Analysis of Plasma at Postoperative Month 1 for Detecting Colorectal Cancer Recurrence.
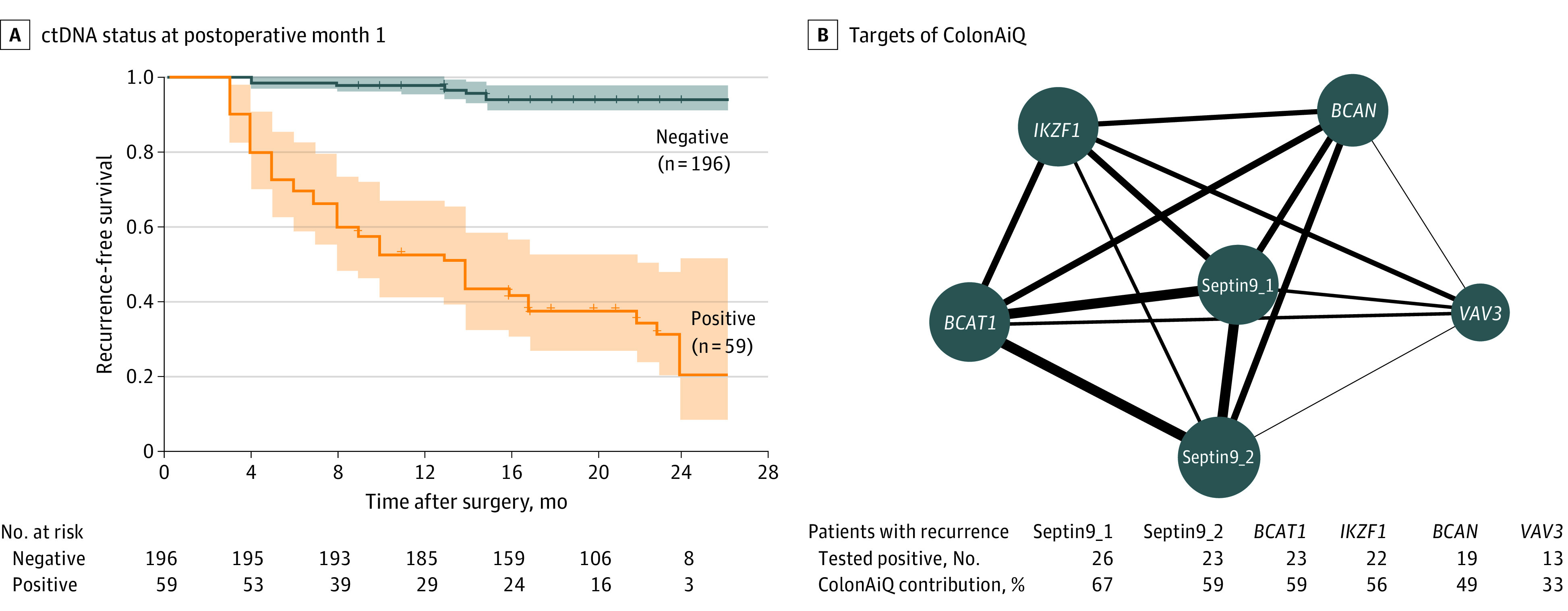
A, Kaplan-Meier estimates of recurrence-free survival, stratified by ctDNA status at postoperative month 1. The recurrence risk for ctDNA-positive patients was significantly higher than that of ctDNA-negative patients (hazard ratio, 17.5; 95% CI, 8.9-34.4; P < .001). P value was determined by the log-rank test. Shaded areas indicate 95% CIs. B, Network map of 6 targets of the ColonAiQ assay28 in patients with recurrence. The size of the nodes is proportional to the number of patients who tested positive, and the thickness of lines is proportional to the number of patients with recurrence who tested positive for 2 targets. Positive rates of 6 targets are shown. BCAN indicates brevican; BCAT1, branched-chain amino acid transaminase 1; IKZF1, ikaros family zinc finger 1; Septin9_1, septin 9 locus 1; Septin9_2, septin 9 locus 2; and VAV3, vav guanine nucleotide exchange factor 3.
