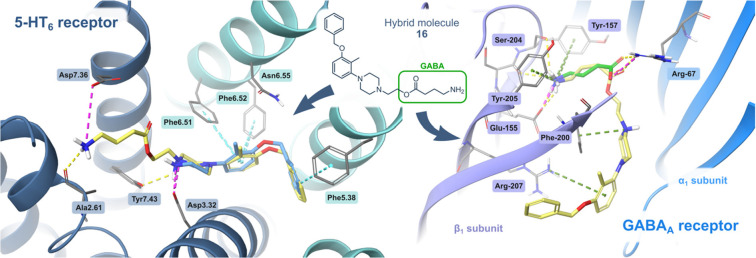
Figure 2.
Potential binding mode of 16 at the orthostatic sites of both targets: left, the 5-HT6 receptor; right, GABA-A receptor (canary, 16; cyan, 5-HT6 receptor ligand 1; green, GABA). 1-[3-(Benzyloxy)-2-methylphenyl]piperazine moiety of 16 established equal interactions to selective 5-HT6 antagonist 1, inside the 5-HT6 receptor. In the GABA-A receptor, the acyl fragment of 16 exhibited a binding pose akin to that of the natural agonist GABA. The remaining hybrid elements did not induce steric hindrance in the active sites of 5-HT6 and GABA-A receptors.