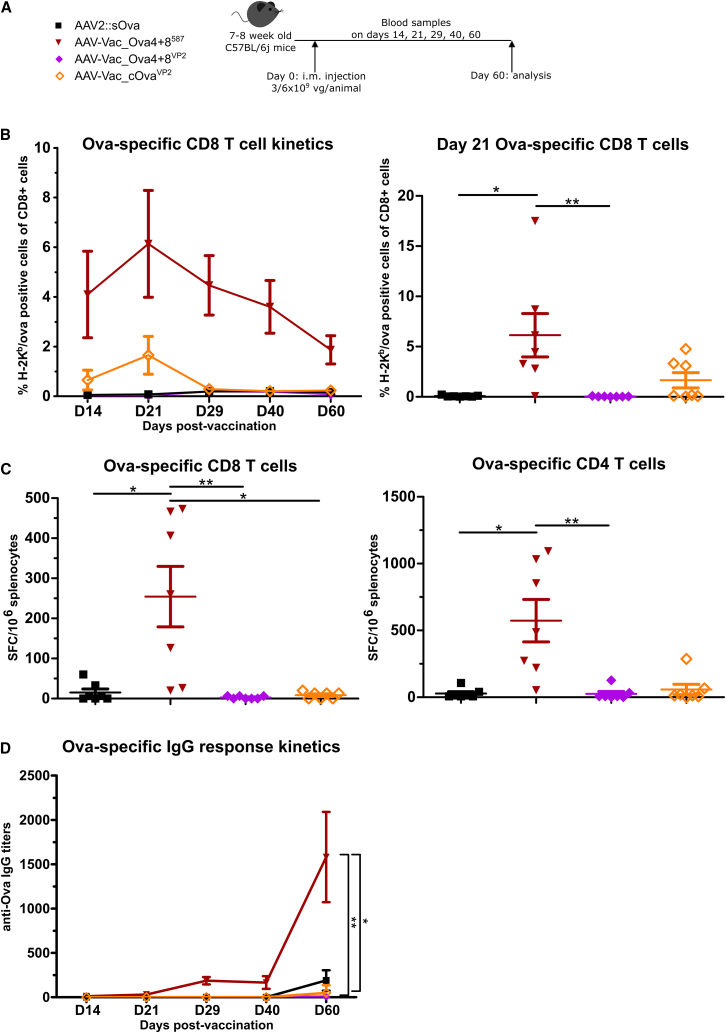
Figure 3.
Display of MHC class I- and MHC class II-restricted Ova epitopes at capsid position I-587 is superior in supporting the induction of adaptive immune responses compared with antigen insertions at the VP2 N terminus
Experimental design as depicted (A). C57BL/6j mice (7 to 8 weeks old; n = 7 per group) received via intramuscular injection AAV2::sOva, AAV-Vac_Ova4+8VP2, or AAV-Vac_cOvaVP2 at a dose of 3 × 109 vector genome-containing particles (vg) per animal, or AAV-Vac_Ova4+8587 at a total dose of 6 × 109 vg per animal. Blood and serum samples were collected on days (D) 14, 21, 28, 40, and 60. Blood samples were evaluated by flow cytometry after staining with H-2Kb/Ova257-264 dextramer for the presence of Ova-specific CD8+ T lymphocytes (B) (D14, Vac_Ova4+8587 n = 6). Splenocytes were isolated at D60 post-vaccine administration and evaluated by ELISpot assays. Quantification of specific T cells was performed by detection of INF-γ secretion upon in vitro re-stimulation with Ova immune-dominant peptides. Cells were re-stimulated with the MHC class I-restricted peptide Ova257-264 to determine the level of anti-Ova CD8+ T cell response (C) (left graph) or with the MHC class II-restricted peptide Ova323-339 to determine the level of anti-Ova CD4+ T cell response (C) (right graph). The number of spot-forming cells (SFCs) per 1 × 106 splenocytes is depicted. Serum samples were evaluated by ELISA for the presence of Ova-specific IgG antibodies (D). D60, Vac_Ova4+8587 and AAV-Vac_Ova4+8VP2 n = 6. Statistical analysis: Kruskal-Wallis test with Dunns post test; ∗p ≤ 0.05, ∗∗p ≤ 0.01. Data are represented as mean with SEM.