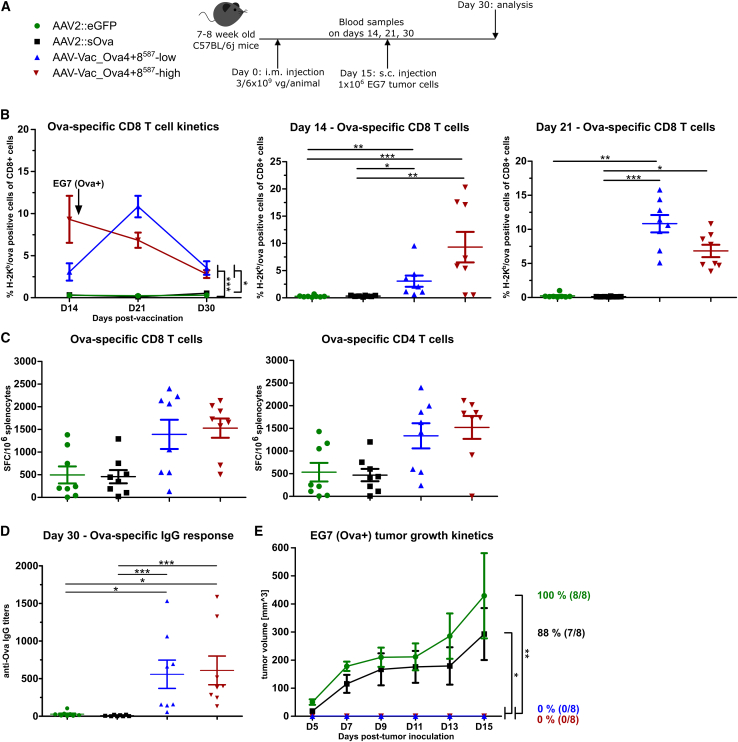
Figure 4.
Antigen-decorated AAV vector-based vaccine-induced Ova-specific immune responses potently inhibit the growth of EG7 tumor cells inoculated 15 days post-vaccination
Experimental design as depicted (A). C57BL/6j mice (7 to 8 weeks old; n = 8 per group) received via intramuscular injection AAV2::sOva or the negative control AAV2::eGFP at a dose of 3 × 109 vector genome-containing particles (vg) per animal, or AAV-Vac_Ova4+8587 at two different doses—either a total dose of 3 × 109 vg per animal (AAV-Vac_Ova4+8587-low) or a total dose of 6 × 109 vg per animal (AAV-Vac_Ova4+8587-high). Fifteen days after vaccination, 1 × 106 Ova-expressing EG7 syngeneic thymoma cells were injected subcutaneously into the flank of each mouse. Blood samples were collected on days (D) 14, 21, and 30, and serum samples on D30. Blood samples were evaluated by flow cytometry after staining with H-2Kb/Ova257-264 dextramer for the presence of Ova-specific CD8+ T lymphocytes (B). Splenocytes were isolated at D30 post-vaccination and evaluated by ELISpot assays for the IFN-γ secretion after in vitro re-stimulation. Cells were re-stimulated with the MHC class I-restricted peptide Ova257-264 to determine the level of anti-Ova CD8+ T cell response (C) (left graph) or with the MHC class II-restricted peptide Ova323-339 to determine the level of anti-Ova CD4+ T cell response (C) (right graph). Serum samples were evaluated by ELISA for the presence of Ova-specific IgG antibodies (D). Tumor growth was measured over time using a digital caliper. The mean tumor volumes in each indicated group as well as the percentage and number of tumor-bearing mice are depicted (E). Statistical analysis: Kruskal-Wallis test with Dunns post test; ∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001. Data are represented as mean with SEM.