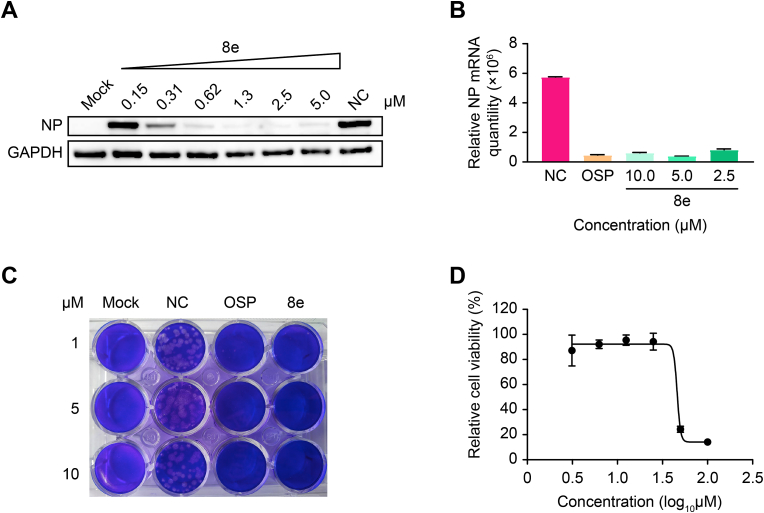
Fig. 4.
In vitroefficacy against H1N1 replication of Compound 8e; OSP was used as the positive control. (A) Western blot assay, (B) qRT–PCR assay and (C) plaque formation assay were used to evaluate the antiviral activity of Compound 8e. The concentration of OSP in the qRT–PCR assay was 5 μM. Mock, blank control; NC, negative control (treated with DMSO). (D) The cytotoxicity of Compound 8e was determined with the CCK-8 reagent.