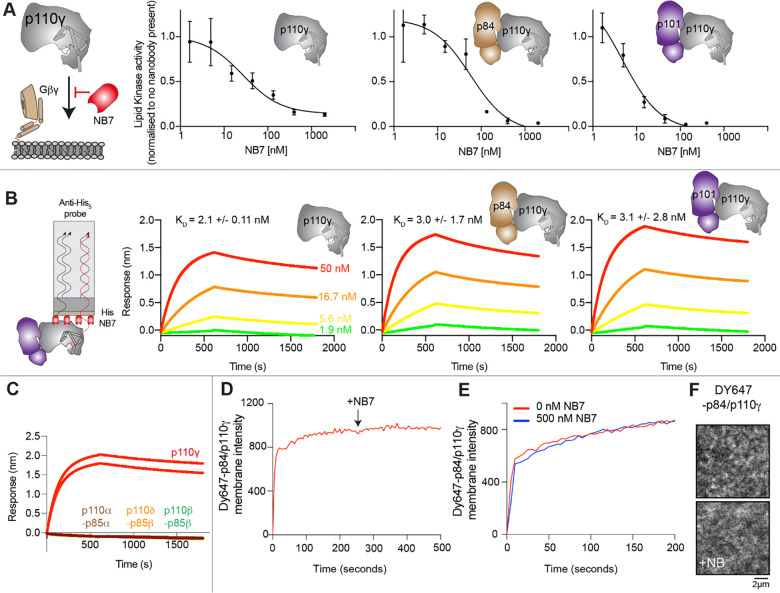
Figure 1. The inhibitory nanobody NB7 binds tightly to all p110γ complexes and inhibits kinase activity, but does not prevent membrane binding.
A. Cartoon schematic depicting nanobody inhibition of activation by lipidated Gβγ (1.5 μM final concentration). Lipid kinase assays show a potent inhibition of lipid kinase activity with increasing concentrations of NB7 (3–3000 nM) for the different complexes. The protein concentration of p110γ (300 nM), p110γ-p84 (330 nM) and p110γ-p101 (12 nM) was different due to intrinsic differences of each complex to be activated by lipidated Gβγ.
B. Association and dissociation curves for the dose response of His-NB7 binding to p110γ, p110γ-p84 and p110γ-p101 (50 – 1.9 nM) is shown. A cartoon schematic of BLI analysis of the binding of immobilized His-NB7 to p110γ is shown on the left. Dissociation constants (KD) were calculated based on a global fit to a 1:1 model for the top three concentrations and averaged with error shown.
B. Association and dissociation curves for His-NB7 binding to p110γ, p110α-p85 α, p110β-p85β, and p110δ-p85β. Experiments were performed in duplicate with a final concentration of 50 nM of each class I PI3K complex.
D. Total Internal Reflection Fluorescence Microscopy (TIRF-M) analysis of the effect of nanobody NB7 on PI3K recruitment to supported lipid bilayers containing H-Ras(GTP) and farnesyl-Gβγ. Y647-p84/p110γ displays rapid equilibration kinetics and is insensitive to the addition of 500 nM nanobody (black arrow, 250 sec) on supported lipid bilayers containing H-Ras(GTP) and farnesyl-Gβγ.
E. Kinetics of 50 nM DY647-p84/p110γ membrane recruitment appears indistinguishable in the absence and presence of nanobody. Prior to sample injection, DY647-p84/p110γ was incubated for 10 minutes with 500 nM nanobody.
F. Representative TIRF-M images showing the localization of 50 nM DY647-p84/p110γ visualized in the absence or presence of 500 nM nanobody (+NB7). Membrane composition for panels C-E: 93% DOPC, 5% DOPS, 2% MCC-PE, Ras(GTP) covalently attached to MCC-PE, and 200 nM farnesyl-Gβγ.