Abstract
Dysbiosis of the gut microbiota with aging contributes to a reduction in important cross-feeding bacterial reactions in the gut and immunosenescence, which could contribute to a decrease in vaccine efficacy. Fever, cough, and fatigue are the main signs of coronavirus disease 2019 (COVID-19); however, some patients with COVID-19 present with gastrointestinal symptoms. COVID-19 vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is one of the best measures to reduce SARS-CoV-2 infection rates and the severity of COVID-19. The immunogenicity of COVID-19 vaccines is influenced by the composition of the gut microbiota, and the immune response to COVID-19 vaccines decreases with age. In this review, we discuss gut microbiota dysbiosis and immunosenescence in the older adults, the role of gut microbiota in improving the efficacy of COVID-19 vaccines, and dietary interventions to improve the efficacy of COVID-19 vaccines in the older adults.
Keywords: COVID-19 vaccine efficacy, Older adults, Gut microbiota, Probiotics, Bifidobacterium
Highlights
-
•
Gut microbiota dysbiosis contributes to immunosenescence in the elderly.
-
•
Altering gut microbiota through dietary interventions can improve vaccine efficacy.
-
•
Dietary interventions may improve COVID-19 vaccine efficacy in the elderly.
1. Introduction
The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is the causative agent of the coronavirus disease 2019 (COVID-19), a pandemic that started in Wuhan, China, in December 2019 [1]. One of the most effective ways to reduce SARS-CoV-2 infection rates and COVID-19 severity is COVID-19 immunization against SARS-CoV-2 [[2], [3], [4]]. However, as the efficacy of COVID-19 vaccines decreases with the emergence of SARS-CoV-2 variants and immunosenescence in the older adults, it is crucial to improve the efficacy of COVID-19 vaccines [5,6]. Adjuvant usage is a successful tactic to increase the immunogenicity of vaccinations in the older adults [7]. However, owing to the side effects of vaccine adjuvants, there is a huge demand for the development of safe vaccine adjuvants [8].
Although COVID-19 typically manifests with respiratory symptoms, gastrointestinal symptoms, such as diarrhea, vomiting, and abdominal discomfort, can occur at high incidence [9]. The angiotensin-converting enzyme 2 (ACE2) receptor interacts with SARS-CoV-2, allowing it to enter host cells [10]. SARS-CoV-2 mainly infects humans through the respiratory tract; however, it can also invade the gastrointestinal tract because ACE2 receptor is expressed in the cells of most human organs [11].
The maturation of immune cells and the normal development of immune function are both facilitated by the gut microbiota, which is crucial for innate and adaptive immunity [12,13]. Gut microbiota dysbiosis, defined as altered composition and decreased diversity of gut microbiota, is common with advancing age. This could contribute to higher rates of SARS-CoV-2 infection and mortality from COVID-19 in the older adults [14,15]. The gut microbiota can influence the adaptive immune response to vaccination [[16], [17], [18], [19]]. Thus, the efficacy of vaccines could be improved with natural adjuvants, such as probiotics targeting the gut microbiota [[20], [21], [22], [23]].
In this review, we summarize the studies on gut microbiota and immunity, gut-lung axis, and interaction between gut microbiota and COVID-19. We provide evidence that alteration of the gut microbiota through dietary interventions could be a promising strategy to improve the efficacy of COVID-19 vaccines in the older adults.
2. Gut microbiota and immunity
2.1. Interaction between gut microbiota and immunity
Microbes exist throughout the human body, including the skin, oral cavity, vagina, respiratory system, and gastrointestinal tract; more than 70% of the human microbiota are present in the gastrointestinal tract. The number of organisms constituting the gut microbiota increases steadily from the stomach to the rectum, estimated at 1013–1014 per gram [24,25]. The composition of the gut microbiota varies with dietary habits and lifestyles [26].
The gut, the body's main immunological organ, contains more than 70% of all immune cells [27]. By interacting with the host's immune system, the gut microbiota plays a critical role in the development of appropriate immunological function [28]. In the absence of microbial stimulus, the gut immune system has difficulty in developing immune functions [[29], [30], [31]]. In germ-free (GF) mice, mucosal immunoglobulin A (IgA) production is induced by gut microbiota colonization. Gut microbiota colonizing GF mice promotes B-cell development, contributing to the establishment of clonal diversity in the B-cell repertoire.
The dysbiosis of gut microbiota is related to immunological imbalances [32,33]. The dysbiosis of gut microbiota in pregnant women with inflammatory bowel disease (IBD) reduces the numbers of class-switched memory B cells and regulatory T cells. In addition, the dysbiosis of gut microbiota is associated with various diseases, such as hepatitis, IBD, cardiovascular diseases, and nervous system disorders [[34], [35], [36]].
2.2. Gut microbiota dysbiosis and immunosenescence in the older adults
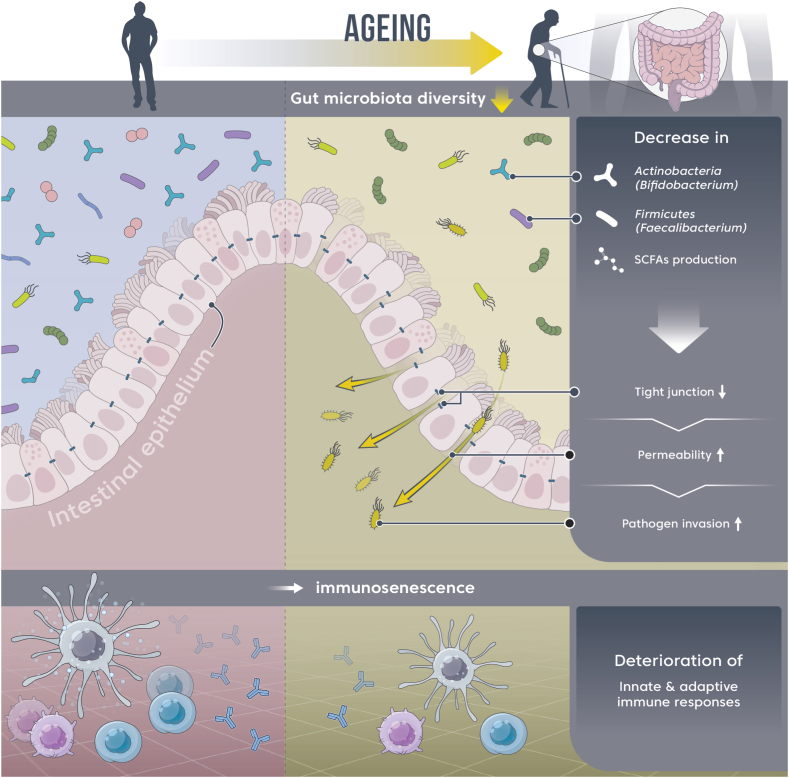
The composition of the human gut microbiota changes with age [37]. This altered gut microbiota contributes to immunosenescence, in which the immune response declines with aging (Fig. 1) [38]. At the phylum level, the relative abundance of Actinobacteria decreases with age [37], and at the genus level, that of Bifidobacterium (belonging to the phylum Actinobacteria) decreases with age. This reduction is presumed to be associated with aging-related immune decline [39]. Bifidobacterium is an early colonizer of the neonatal gut and is predominant in infants during lactation. This could have beneficial effects on host health through immunomodulation [40].
Fig. 1.
Aging-induced gut microbiota dysbiosis and decreased immune responses.
Ingestion of Bifidobacterium as probiotics improves both innate and acquired immunity, as seen in Table 1. Depending on their interaction with microbes in the gastrointestinal barrier, they can trigger either a normal immune response or the development of an infection. Tight junctions between intestinal epithelial cells can maintain the integrity of the intestinal barrier, and the ingestion of Bifidobacterium can improve intestinal epithelial tight junction barrier and increase mucus layer thickness [[41], [42], [43]]. Bifidobacterium bifidum enhanced the intestinal epithelial tight junction barrier in a Toll-like receptor-2 pathway-dependent manner in Caco-2 cells. Bifidobacterium breve B-3 improved intestinal tight junction barrier integrity by the transcriptional regulation of claudin-4 in Caco-2 cells. Bifidobacterium pseudolongum Patronus altered gut microbiota and increased mucus layer thicknesses in rats. Viral infection in the human body involves several steps, including the attachment of virions to the cell surface. The most common routes of viral infection are the respiratory and digestive tracts. Beneficial bacteria, such as Bifidobacterium, are involved in preventing viral entry into the human body [[44], [45], [46], [47], [48]]. Bifidobacterium thermophilum RBL67 inhibits the binding of rotavirus to Caco-2 and HT-29 cells. Bifidobacterium adolescentis LMG10502 inhibits the binding of norovirus to Caco-2 and HT-29 cells. The inhibitory effect of microbiota against virus invasion into cells is observed across various mammalian cells. Bifidobacterium infantis MCC12 and B. breve MCC1274 reduce the binding of rotavirus to porcine intestinal epithelial cells. B. breve DSM20091 and Bifidobacterium longum Q46 directly bind to vesicular stomatitis virus, prevent its adsorption and internalization into porcine intestinal and lung cells, and produce metabolites with antiviral effects. Therefore, the dietary intake of Bifidobacterium, which reduces with aging, could inhibit the entry of viruses into the human body.
Table 1.
Improvement of innate and adaptive immunity through dietary Bifidobacterium intake as a probiotic.
| Probiotics | Host | Immune response | References |
|---|---|---|---|
| Innate immunity: Improving tight junction barrier and strengthening the mucus layer | |||
| · B. bifidum | · Human cell | · Enhancement of the intestinal epithelial tight junction barrier in a Toll-like receptor-2 (TLR-2) pathway-dependent manner | [41] |
| · B. breve B-3 | · Human cell | · Improving intestinal tight junction barrier integrity by transcriptional regulation of claudin-4 | [42] |
| · B. pseudolongum Patronus | · Rat | · Alterations in gut microbiota and increases in mucus layer thickness | [43] |
| Innate immunity: Inhibition of viral invasion | |||
| · B. thermophilum RBL67 | · Human cell | · Reduced entry into epithelial cells by binding to rotavirus | [45] |
| · B. adolescentis LMG10502 | · Human cell | · Reduced entry into epithelial cells by binding to norovirus | [47] |
| · B. infantis MCC12, B. breve MCC1274 | · Pig cell | · Reduced entry into epithelial cells by binding to rotavirus | [46] |
| · B. breve DSM20091, B. longum Q46 | · Pig cell | · Reduced entry into epithelial cells by binding to vesicular stomatitis virus | [48] |
| · Production of metabolites with antiviral effects | |||
| Innate immunity: Monocytes, granulocytes, NK cells, and cytokines | |||
| · B. lactis HN019, B. lactis Bi-07 | · Human | · Enhancement of natural killer (NK) cell, monocyte, and granulocyte activities | [50,51] |
| · B. longum BB536 | · Human | · Enhancement of NK cell and neutrophil activities | [52] |
| · B. infantis CCUG52486, B. longum SP07/3 | · Human | · Enhancement of NK cell activity | [54] |
| · B. adolescentis ATCC15704, B. breve ATCC15700, B. longum ATCC15707 | · Rat | · Significant increase in total white blood cell count | [53] |
| · Significant increase in the level of IL-8 that attracts and activates monocytes | |||
| Adaptive immunity: Cell-mediated reaction: T lymphocytes and B lymphocytes | |||
| · B. longum BB536 | · Human | · Improvement of Th1 immune response by increasing the number of IFN-γ secreting cells | [55] |
| · B. breve Yakult | · Mice | · Induces the development of IL-10-producing Tr1 cells in the large intestine by activating intestinal dendritic cells | [56] |
| · B. lactis HN019 | · Human | · Increase in the number of helper T cells (Th) | [50] |
| · B. longum AH1206 | · Mice | · Increase in the number of regulatory T cells (Treg) | [57] |
| · B. bifidum PRI1 | · Mice | · Improvement of Treg functionality | [58] |
| Adaptive immunity: Humoral reaction: Antibodies | |||
| · B. adolescentis ATCC15704, B. breve ATCC15700, B. longum ATCC15707 | · Rat | · Significant increase in the level of IgA, the major antibody in the intestine | [53] |
| · B. longum BB536 | · Human | · Significant increase in the level of IgA, the major antibody in the intestine | [59] |
The factors contributing to the decreased immunity in the older adults include the decrease in the function of innate immunity [49]. Probiotics are living microorganisms that, when consumed in moderation, have beneficial health effects on the host. Ingesting Bifidobacterium as probiotics could enhance cellular immunity in the innate immune system in senile rats as well as in the older adults [[50], [51], [52], [53], [54]]. Bifidobacterium lactis HN019 and B. lactis Bi-07 restored innate immunity by enhancing the activities of natural killer (NK) cells, monocytes, and granulocytes. B. longum BB536 significantly increases the activities of NK cells and neutrophils in the older adults. B. infantis CCUG52486 and B. longum SP07/3 considerably improve the decreased NK cell activity in the older adults. The effect of probiotic Bifidobacterium on the innate immune response was confirmed not only in rats but also in humans. B. adolescentis ATCC15704, B. breve ATCC15700, and B. longum ATCC15707 significantly increase the decreased total leukocyte count in aging rats. The increase in the leukocyte count induced by B. adolescentis intake could be attributed to the increase in neutrophil and monocyte counts. The level of IL-8, a chemokine that recruits and activates neutrophils and monocytes, was significantly decreased in aging rats. The intake of B. adolescentis restored IL-8 levels to normal levels.
The genus Bifidobacterium exhibits immune effects in innate immunity as well as in cell-mediated response in adaptive immunity [50,53,[55], [56], [57], [58], [59]]. B. longum BB536 improved the Th1 immune response by increasing the number of IFN-γ-secreting cells in healthy infants. B. breve Yakult induced the development of IL-10-producing Tr1 cells in the colon by activating intestinal dendritic cells in mice. B. lactis HN019 increases the number of helper T cells in the older adults. In aging mice, B. longum AH1206 induces a protective effect against respiratory allergic inflammation by increasing the number of regulatory T cells. B. bifidum enhances the function of intestinal regulatory T cells by promoting the IL-10/IL-10Rα self-stimulatory loop.
The human B-cell cluster decreases both quantitatively and qualitatively in the older adults, with a decrease in the number of B cells as well as in their ability to produce antibodies [60]. Within the adaptive immune response, the ability to produce antibodies, a humoral response, is enhanced by the genus Bifidobacterium [53,59]. The level of IgA, a major intestinal antibody, is significantly increased in aged rats, with the ingestion of B. adolescentis ATCC15704, B. breve ATCC15700, and B. longum ATCC15707; in addition, B. longum BB536 increases serum IgA levels in the older adults.
The decreased immune function in the older adults is associated with lower levels of short-chain fatty acids (SCFAs) [61], which could be attributed to the low abundance of SCFA-producing bacteria [62,63]. Gut microbiota dysbiosis due to a decrease in the abundance of SCFA-producing bacteria, such as Faecalibacterium prausnitzii, Roseburia faecis, Anaerostipes butyraticus, and Ruminococcaceae, with aging contributes to a decrease in immune function, such as decreased immune cell activity.
3. Gut-lung axis
3.1. Bidirectional interaction between the gut and the lung
Although the gut and the lung are anatomically distinct, there is a bidirectional crosstalk between the respiratory tract and gastrointestinal tract. The connection between the two organs is called the gut-lung axis [64]. Chronic respiratory diseases and respiratory viral infections are correlated with gastrointestinal diseases [[65], [66], [67], [68], [69], [70]]. Patients with chronic gastrointestinal disorders, such as irritable bowel syndrome (IBS) and IBD, are more likely to develop chronic lung diseases, such as asthma and chronic obstructive pulmonary disease (COPD). In addition, gastrointestinal symptoms, such as diarrhea and vomiting, are common symptoms of respiratory viral infections. IBS increases the risk of chronic lung diseases, such as asthma and COPD.
Absence or dysbiosis of gut microbiota contributes to several lung diseases by affecting both intestinal and pulmonary immunity [[71], [72], [73]]. Correlations between lung diseases and dysbiosis of gut microbiota have been reported widely [[74], [75], [76], [77], [78], [79], [80]]. Early exposure to the gut microbiota is essential for the development and maturation of the immune system, and the absence of gut microbiota may increase susceptibility to asthma. There is a decrease in gut microbiota diversity in patients with asthma, and a low diversity of the gut microbiota in infancy is correlated with asthma in childhood. In addition, there are specific alterations in the composition of the gut microbiota in patients with asthma. The abundance of the butyrate-producing bacterium F. prausnitzii was decreased, whereas that of Clostridium and Eggerthella lenta was increased in individuals with asthma. Similarly, a lower relative abundance of SCFA-producing bacteria, such as the genera Bifidobacterium, Akkermansia, and Faecalibacterium, and a higher relative abundance of particular fungi (Candida and Rhodotorula) increase the incidence of atopy and asthma in childhood. According to a clinical study, patients with COPD and healthy controls have distinct gut microbiota compositions. In addition, non-clinical studies have suggested that the gut microbiota significantly influences the development of cigarette smoking-induced COPD. A non-clinical study showed that the transplantation of gut microbiota from healthy controls and the symbiotic bacterium Parabacteroides goldsteinii restored gut health in patients with COPD. Cystic fibrosis (CF) is another chronic lung disease that affects the gastrointestinal tract, resulting in intestinal inflammation. Dysbiosis of the gut microbiota plays a role in the etiology of intestinal inflammation in CF.
3.2. Association between gut microbiota and lung diseases in the older adults
The prevalence of lung diseases, such as COPD, asthma, and tuberculosis, is high among the older adults, who are particularly susceptible to lung infections [81]. The incidence of respiratory tract infections increase significantly in the older adults owing to immunosenescence with advancing age; this contributes to the mortality from these infections [82].
The dysbiosis of the gut microbiota in the older adults affects not only intestinal immunity but also pulmonary immunity, thus contributing to lung-related diseases [83]. Age-related alterations in gut microbiota contribute to the pathogenesis of allergic airway diseases. As there are few studies on respiratory infections, further research is needed to determine whether dysbiosis of the gut microbiota caused by aging increases the susceptibility to respiratory infections.
4. Gut microbiota as a natural adjuvant for respiratory vaccines
Vaccination is the most effective method to protect against infections. However, live vaccines have serious side effects. Therefore, vaccines currently on the market are mostly inactivated or contain only pathogenic components and thus with low immunostimulatory potential. Vaccine efficacy varies for each individual depending on age, genetics, sex, and environmental factors [[84], [85], [86], [87]]. Age has a major influence on the vaccine response. With age, the ability to develop an immune response following vaccination and the resistance against infections decrease gradually, leading to a reduction in vaccine efficacy in the older adults [[88], [89], [90], [91]]. Influenza vaccine efficacy is lower in the older adults than in young adults, which could be attributed to the reduction in antibody production and T cell immune response owing to the reduced migration of dendritic cells to draining lymph nodes. Therefore, it is crucial to alleviate the reduction in vaccine immune response in the older adults.
High-dose vaccination and the use of adjuvants could improve the decrease in vaccine immune responses due to aging. Appropriate use of adjuvants enhances the rate and magnitude of immune responses against antigens in the vaccine [[92], [93], [94], [95]]. Aluminum salt-based vaccine adjuvants are used as adjuvants for diphtheria, tetanus, and pertussis (DTaP), haemophilus influenzae B (Hib), pneumococcal, and hepatitis A and B vaccines. In addition, the use of virosome as an adjuvant in influenza vaccines stimulates a strong antibody response and activates T lymphocytes. An oil-in-water emulsion adjuvant enhances the efficacy of influenza vaccines in children. However, aluminum salt-based vaccine adjuvants can cause side effects [[96], [97], [98]]. Aluminum salt-based vaccine adjuvants can have serious and widespread adverse effects on health, as they carry the risk of autoimmunity, long-term brain inflammation, neurological deficits similar to those in Alzheimer's disease in adults, and autism in children. Therefore, there is a high demand for the development of a safe adjuvant considering the side effects of artificial vaccine adjuvants.
The gut microbiota affects not only the innate immunity of the host, but also the immunity against infections. It influences the immune response of respiratory vaccines, exhibiting potential as a natural adjuvant for respiratory vaccines [21,[99], [100], [101], [102]]. In addition, the decrease in vaccine efficacy due to aging can be restored by improving immunosenescence through the regulation of the gut microbiota [[103], [104], [105]]. As indicated in Table 2, the gut microbiota can be modulated by dietary probiotic intake, which enhances the efficacy of respiratory vaccines. Antigen-specific antibody titers increased after influenza vaccination in a group of people aged 70 years or older that consumed a mixture of Lactobacillus paracasei, Streptococcus thermophilus, and L. bulgaricus. Similarly, antigen-specific IgG and IgA antibody levels were significantly increased in a group of people aged 65−85 years taking L. plantarum CECT7315/7316; in addition, IgM antibody levels were increased although not significantly. Ingesting a mixture of L. plantarum PBS067, B. animalis subsp. lactis BL050, B. longum subsp. infantis BI221, and B. longum subsp. longum BLG240 improves the efficacy of vaccines by regulating the composition of the gut microbiota in older adults people aged 60−80 years, improving the immune response and lowering the incidence of influenza symptoms.
Table 2.
Mechanism of respiratory vaccine efficacy improvement via gut microbiota regulation through dietary probiotic intake.
| Probiotics | Dose | Vaccine | Study design and mechanisms to improve respiratory vaccine efficacy | References |
|---|---|---|---|---|
| Study individual age: Infant (Human) | ||||
| · B. bifidum DSMZ20082, B. infantis ATCC15697, B. longum ATCC157078, L. acidophilus ATCC4356 | · 3 × 109 CFU per strain | · Measles, mumps, rubella, and varicella (MMRV) attenuated live vaccine | · MMRV vaccination after taking probiotics for 2 months | [100] |
| · Continue taking probiotics for up to 3 months after vaccination | ||||
| · In the group taking probiotics, IgG antibody levels increased, but not significantly | ||||
| · L. paracasei F19 | · 108–1010 CFU | · Diphtheria, tetanus, pertussis (DTaP)- polio (IPV)- Haemophilus influenzae type B (Hib)-conjugate vaccine | · Daily probiotic intake from 4th month to 13th month | [101] |
| · 1st dose at 3rd month, 2nd dose at 5th month, 3rd dose at 12th month | ||||
| · Significantly increased IgG antibody levels against diphtheria in the group taking probiotics | ||||
| Study individual age: Adult (Human) | ||||
| · B. animalis ssp. lactis BB-12,L. paracasei ssp. paracasei 431 | · 1 × 109 CFU | · Inactivated trivalent influenza vaccine | · Increased antigen-specific IgG and IgA levels | [172] |
| · Increased seroconversion rates for IgG | ||||
| · L. GG ATCC53101 | ·1 × 1010 CFU | · Trivalent influenza attenuated live vaccine | · Taking probiotics twice a day for 28 days after vaccination | [21] |
| · Increased levels of antibodies against H3N2 antigen in the Lactobacillus GG group | ||||
| · L. fermentum CECT5716 | · 1 × 1010 CFU | · Inactivated influenza vaccine | · Daily probiotic intake for 2 weeks each before and after vaccination | [102] |
| · Increased antigen-specific IgA and total IgM concentrations in the probiotic group | ||||
| Study individual age: Older adults (Human) | ||||
| · L. paracasei DN-114 001, L. bulgaricus, Streptococcus thermophilus | · 2 × 1010 CFU | · Trivalent inactivated influenza vaccine | · Daily probiotic intake from 4 weeks before vaccination to 9 weeks after vaccination | [103] |
| · Increased B antigen-specific antibody IgG titer in the group taking probiotics | ||||
| · L. plantarum CECT7315/7316 | · 5 × 108–109 CFU | · Trivalent influenza vaccine | · Daily probiotic intake for 3–4 months after vaccination | [104] |
| · In the group taking probiotics, antigen-specific IgG and IgA antibody levels increased significantly, and IgM antibody levels increased, although not significantly | ||||
| · L. plantarum PBS067, B. animalis subsp. lactis BL050, B. longum subsp. infantis BI221, B. longum subsp. longum BLG240 | · 1 × 109 CFU per strain | · Influenza vaccine | · Taking probiotics for 28 days after vaccination | [105] |
| · Probiotic intake improves the immune system and reduces the incidence of influenza symptoms by regulating the gut microbiota composition | ||||
5. Interaction of gut microbiota and COVID-19
5.1. Gut microbiota dysbiosis and COVID-19
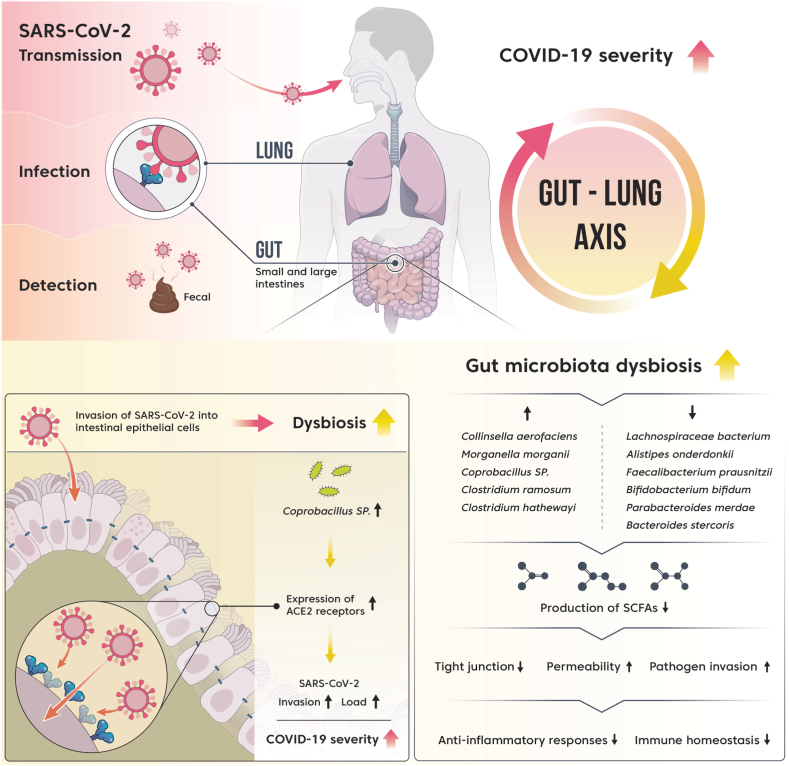
SARS-CoV-2 RNA was detected in the fecal samples of patients with COVID-19, suggesting SARS-CoV-2 infection in the gut [106] (Fig. 2, upper left). ACE2 is a cellular receptor used by SARS-CoV-2 to enter human cells. Transmembrane serine protease (TMPRSS2) plays a priming role in the entry of SARS-CoV-2 into host cells through the cleavage of the spike protein of SARS-CoV-2 [107,108]. A high co-expression of ACE2 receptor and TMPRSS2 was detected in the gut as well as in the lung, providing evidence that SARS-CoV-2 can infect the gastrointestinal tract [109] (Fig. 2, upper left). In particular, the expression of ACE2 and TMPRSS2 was the highest in the small intestine compared with that in other tissues [[110], [111], [112]].
Fig. 2.
Interaction between gut microbiota dysbiosis and COVID-19 severity.
In most clinical studies of COVID-19, the main symptoms are fever, cough, and fatigue [[113], [114], [115]]. However, gastrointestinal SARS-CoV-2 infection through ACE2 receptors on gastrointestinal epithelial cells causes COVID-19 in some patients who exhibit gastrointestinal symptoms [[115], [116], [117]]. In a study on the clinical symptoms of COVID-19, majority of the 204 patients with COVID-19 visited the hospital with fever or respiratory symptoms. However, 103 patients (50.5%) showed gastrointestinal symptoms. In a meta-analysis, the prevalence of gastrointestinal symptoms was 17.6% (95% confidence interval [CI], 12.3–24.5) in 4243 patients with COVID-19. Anorexia (26.8%) was the most prevalent gastrointestinal symptom, followed by diarrhea (12.5%), nausea/vomiting (10.2%), and abdominal pain/discomfort (9.2%). These gastrointestinal symptoms could be attributed to the SARS-CoV-2 infection from the lungs; the virus crosses the mucosal immune barrier and affects the gut along the “gut-lung axis.”
The rate of detection of viral RNA and the viral load in feces were higher in patients with diarrhea than in patients without diarrhea [117]. In addition, the gastrointestinal symptoms caused by SARS-CoV-2 are highly correlated with the severity of COVID-19 [117,118]. In a meta-analysis of 4243 patients with COVID-19, 17.1% (95% CI, 6.9–36.7) of patients with severe COVID-19 and 11.8% (95% CI, 4.1–29.1) of patients with non-severe COVID-19 had gastrointestinal symptoms. In a meta-analysis focused on the correlation between gastrointestinal symptoms and severity of COVID-19, more than 40% of patients with COVID-19 with gastrointestinal symptoms showed severity; in addition, abdominal pain increased the risk of COVID-19 severity by almost 2.8 times.
SARS-CoV-2 infection causes dysbiosis of the gut microbiota in patients with COVID-19 [[119], [120], [121], [122], [123]]. Patients with COVID-19 have lower diversity and richness of gut microbiota than healthy people. The gut microbiota of patients with COVID-19 shows an increased relative abundance of opportunistic pathogens, such as Streptococcus, Rothia, Veillonella, Erysipelatoclostridium, and Actinomyces, harmful bacteria, such as Clostridium hathewayi and Actinomyces viscosus, and pathogens associated with Crohn's disease (Ruminococcus gnocavus and Ruminococcus torques), compared with that in healthy controls. The relative abundance of beneficial bacteria, such as F. prausnitzii, Eubacterium rectale, and bifidobacteria with immunomodulatory potential was less. F. prausnitzii induces priming of human colon regulatory T cells, which secrete the anti-inflammatory cytokine, IL-10, and is a major producer of SCFAs.
The dysbiosis of gut microbiota, such as an increase in the relative abundance of pathogenic bacteria and a decrease in the relative abundance of beneficial bacteria, contributes to an abnormal immune response and severity of COVID-19 (Fig. 2) [121,122]. There is a positive correlation between the severity of COVID-19 and the genera Coprobacillus, Clostridium ramosum, and C. hathewayi. Both C. ramosum and C. hathewayi are associated with human infections and bacteremia, and the genus Coprobacillus strongly upregulates the expression of ACE2 in the gut. In contrast, Lachnospiraceae bacterium 5_1_63FAA, Alistipes onderdonkii, and F. prausnitzii show a negative correlation with COVID-19 severity. The genus Alistipes is involved in the maintenance of intestinal immune homeostasis, and F. prausnitzii has anti-inflammatory properties [122]. F. prausnitzii and B. bifidum show a negative correlation with severity after adjusting for confounding factors, including age and use of antibiotics. F. prausnitzii is a butyrate-producing bacterium. Butyrate improves the regeneration and integrity of the epithelial barrier and promotes the secretion of mucin and the antimicrobial peptide, defensin, thereby reducing the rate of respiratory viral infections [[124], [125], [126]]. In addition, butyrate exerts anti-inflammatory effects through several mechanisms [127].
The correlation between the gut microbiota and the severity of COVID-19 was confirmed indirectly (Fig. 2) [123]. When comparing the gut microbiota composition between feces with high SARS-CoV-2 infectivity and that with low or no SARS-CoV-2 infectivity, the opportunistic pathogens Collinsella aerofaciens and Morganella morganii were more abundant in the fecal samples with high SARS-CoV-2 infectivity. C. aerofaciens is an inflammatory bacterium [128], and M. morganii is an opportunistic pathogen capable of causing serious infections in immunocompromised hosts, such as neonates [129]. SCFA-producing microorganisms were more abundant in fecal samples with low or no SARS-CoV-2 infectivity, such as Parabacteroides merdae, Bacteroides stercoris, A. onderdonkii, and Lachnospiraceae bacterium 1_1_57FAA. B. stercoris downregulates the expression of ACE2 in the gut, suggesting that this could interfere with the entry of SARS-CoV-2 into intestinal cells [123].
Among SARS-CoV-2 cases, the older adults have high rates of infection, hospitalization, and mortality [130,131]. Patients with severe COVID-19 are significantly older and have a higher frequency of abdominal pain than patients with non-severe COVID-19 [[132], [133], [134]]. The dysbiosis of the gut microbiota in the older adults [135] could have contributed to the severity of COVID-19 in them; however, additional studies are warranted.
5.2. Potential of gut microbiota in improving the efficacy of COVID-19 vaccines
Patients with IBD exhibit a lower immune response following COVID-19 vaccination than healthy controls [136,137]. The composition of the gut microbiota correlates with the immunogenicity of COVID-19 vaccines [138]. The higher the relative abundance of B. adolescentis, the higher the antibody response to COVID-19 vaccines, whereas the higher the relative abundance of Bacteroides vulgatus, Bacteroides thetaiotaomicron, and Ruminococcus gnavus, the lower the antibody response.
The level of anti-SARS-CoV-2 S1 RBD IgG antibody elicited in response to COVID-19 vaccination varies significantly with age; the antibody response is lower in the older adults [139,140]. The immune responses to mRNA vaccines in groups younger than 60 and older than 80 years were compared; significantly lower antibody titers were observed in the older group. Even in the real world, the effectiveness of COVID-19 vaccines is lower in older people [141]. Among 2,828,294 participants, the effectiveness of COVID-19 vaccines in preventing death after hospitalization due to COVID-19 was 91.0% (89.0–92.6) in those aged 60–69 years, 85.0% (83.1–86.7) in those aged 70–79, and 68.4% (65.7–70.9) in those over 80 years of age.
In the older adults, the decrease in the adaptive immune system function due to aging reduces the immune response elicited by COVID-19 vaccines. Therefore, the efficacy of COVID-19 vaccines has age-dependent limitations [140,[142], [143], [144], [145], [146], [147]]. COVID-19 severity is associated with lymphopenia and low diversity of the T cell receptor (TCR) for the SARS-CoV-2 epitope. Immunosenescence occurs in the older adults, accompanied by a decrease in the naive TCR repertoire and a decrease in the number of naive T cells. This could contribute to the severity of SARS-CoV-2 infection in the older adults despite vaccination. In addition, the neutralizing antibody titer after the second dose of COVID-19 vaccines is lower in the older adults than in the adult group. Immunosenescence through reduced B-cell response is another potential cause for the high SARS-CoV-2 infection rates and severe COVID-19. Gut microbiota dysbiosis could be involved in the decrease in COVID-19 vaccine efficacy in the older adults; however, further studies are required. Gut microbiota regulators are used as adjuvants in vaccines for other respiratory infections; this could also be considered for COVID-19 vaccines.
6. Dietary interventions to improve COVID-19 vaccine efficacy in the older adults
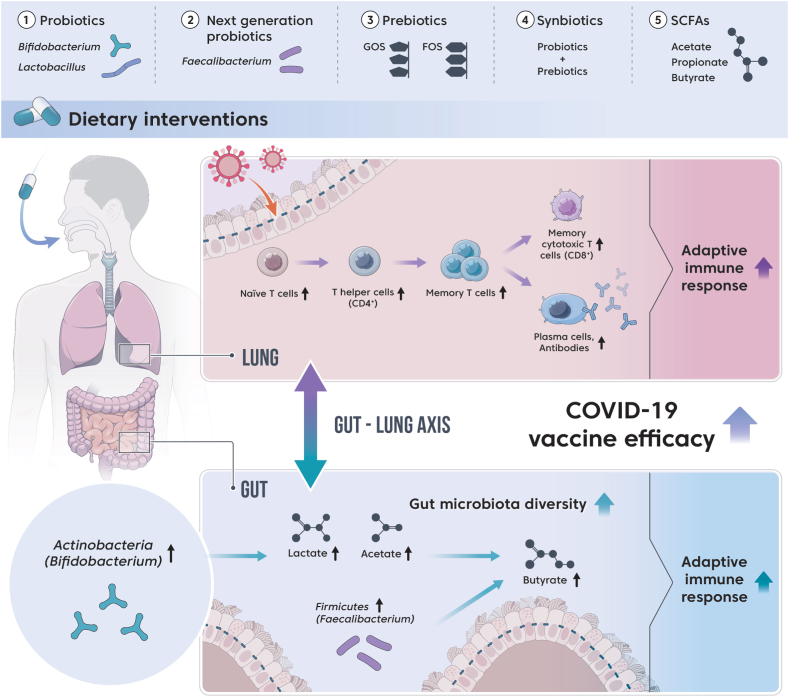
The efficacy of various respiratory vaccines is improved through the modulation of gut microbiota. Therefore, the modulation of gut microbiota through dietary intervention could alleviate the severity and reduce mortality in older adults patients with COVID-19 after vaccination (Fig. 3). Table 3 presents dietary interventions that can improve COVID-19 vaccine efficacy in the older adults by altering the gut microbiota.
Fig. 3.
Dietary interventions to improve COVID-19 vaccine efficacy by modulating the gut microbiota in the older adults.
Table 3.
Dietary interventions to improve COVID-19 vaccine efficacy in the older adults by modulating the gut microbiota.
| Dietary interventions | Study individual | Intervention period | Mechanism of improving the efficacy of COVID-19 vaccines | References |
|---|---|---|---|---|
| Probiotics and next generation probiotics | ||||
| · 1:1 mixture of B. longum Bar33 + L. helveticus Bar13 1 × 109 CFU | · Older adults over 75 years old (84.6 ± 7.8 years old) | · 30 days | · Significant increase in the numbers of B cells and naive T cells | [148] |
| · Significant increase in the number of CD8+-activated memory T cells | ||||
| · B. longum BB536 5 × 1010 CFU twice/day | · Older adults over 75 years old (81.7 ± 8.7 years old) | · 12 weeks | · Increased abundance of bifidobacteria in gut microbiota composition | [59] |
| · Increased titer of specific antibodies against influenza A/H1N1 antigen | ||||
| · L. plantarum P-8 6 × 1010 CFU | · Adults and older adults | · 4 weeks | · Increased abundance of bifidobacteria in gut microbiota composition | [149,150] |
| · L. paracasei MCC1849 1 × 1010 CFU/day | · Older adults over 65 years old | · 12 weeks | · Increased titers of specific antibodies against influenza A/H1N1 and B antigens | |
| · L. delbrueckii subsp. bulgaricus 8481 + Streptococcus thermophilus NBIMCC No.8357 3 × 107 CFU three times/day | · Older adults over 65 years old | · 6 months | · Increased number of immature T cells that are potential responders to new antigens | [151] |
| · Slowed down aging of T cell subpopulation | ||||
| · Faecalibacterium prausnitzii | · Increased production of the metabolite butyrate | [152,153] | ||
| Prebiotics and synbiotics | ||||
| · Prebiotics [Galactooligosaccharides (GOS) 5.5 g/day] | · Older adults (69.3 ± 4.0 years old) | · 4 weeks | · Significant increase in the abundance of beneficial bacteria, especially bifidobacteria, in the gut microbiota | [[155], [156], [157]] |
| · Prebiotics [GOS (0.4 g/100 kcal) + bifidogenic growth stimulator (1.65 μg/100 kcal) + fermented milk] | · Older adults (79.9 ± 9.5 years old) | · 14 weeks | · Long-term maintenance of antibody titer against viral A/H1N1 antigen through improvement of gut microbiota dysbiosis | |
| · Synbiotics (3.5 × 1010 CFU B. bifidum BB-02 + 3.5 × 1010 CFU B. lactis BL-01 + fructooligosaccharide) | · Older adults: control group 71 years old (63–85 years old), treatment group 73 years old (68–90 years old) | · 8 weeks | · Significant increase in the abundance of bifidobacteria and lactobacilli in the gut microbiota composition | [159] |
| · Synbiotics [2 × 1011 CFU B. longum + 6 g of prebiotic (inulin + oligofructose)] | · Older adults (71.9 ± 5.4 years old) | · 4 weeks | · Significant increase in the abundance of bifidobacteria in the gut microbiota | [160] |
| · Abundance of Actinobacteria and Firmicutes increased but that of Proteobacteria decreased | ||||
| · Increase in butyrate production | ||||
6.1. Traditional probiotics
Ingestion of the genus Bifidobacterium as probiotics will increase the number of Bifidobacterium cells in the gut, thereby improving adaptive immunity [59,148] in the older adults. Ingestion of a mixture of B. longum Bar33 and Lactobacillus helveticus Bar13 does not significantly alter the number of total Th cells (CD4+) or Tc cells (CD8+) in the older adults; however, it significantly increases the number of B cells and naive T cells. In addition, it increases the number of CD8+ activated memory T cells, contributing to the improvement of adaptive immunity. Ingestion of B. longum BB536 increased the abundance of bifidobacteria in the gut microbiota and serum IgA levels in the older adults, demonstrating its potential for regulating the adaptive immune function by improving the gut microbiota.
In addition to Bifidobacterium, it has been reported that the ingestion of Lactobacillus, which is widely used as probiotics, increases the number of Bifidobacterium cells [149] and improves adaptive immunity [150,151]. Influenza antigen-specific antibody levels were significantly increased when heat-treated L. paracasei MCC1849 was consumed by the older adults aged 85 years or older. In addition, the ingestion of Lactobacillus delbrueckii subsp. bulgaricus 8481 conjugated with Streptococcus thermophillus NBIMCC No.8357 in the older adults aged 65 years or older increases the number of immature T cells, potential responders to novel antigens, and slows the aging of T-cell subpopulations.
6.2. Next-generation probiotics
Traditional probiotics, such as Bifidobacterium and Lactobacillus, do not target specific diseases. However, next-generation probiotics target specific diseases based on next generation sequencing and bioinformatics. F. prausnitzii, Akkermansia muciniphila, Bacteroides fragilis, and some Clostridium are included in next-generation probiotics [152]. The abundance of F. prausnitzii, a butyrate-producing bacterium, is greatly reduced in the gut of the older adults [153]. Therefore, dietary intake of F. prausnitzii as a next-generation probiotic could improve adaptive immunity in the older adults. However, as there are few clinical studies on the use of next-generation probiotics as probiotic formulation, further studies focusing on safety aspects are necessary.
6.3. Prebiotics
The intake of prebiotics that selectively promote the growth of beneficial bacteria among the gut microbiota is another dietary strategy for improving the health of the host; most dietary fibers are classified as prebiotics [154]. Galactooligosaccharides (GOS) and fructooligosaccharides (FOS) are included in prebiotics. The ingestion of prebiotics significantly increases the abundance of beneficial bacteria, particularly bifidobacteria, in the older adults, and improves the immune response of virus vaccines by modulating the gut microbiota [[155], [156], [157]]. Ingestion of GOS significantly increases the abundance of bifidobacteria in the gut microbiota and maintains the antibody titer against the viral A/H1N1 antigen for a long time through enhancing the gut microbiota.
6.4. Synbiotics
The ingestion of synbiotics, a mixture of probiotics and prebiotics, increases the abundance of beneficial bacteria in the gut microbiota and levels of metabolites in the older adults [[158], [159], [160]]. The ingestion of synbiotics (B. bifidum BB-02 + B. lactis BL-01 + FOS) significantly increases the abundance of bifidobacteria and lactobacilli in the gut microbiota. In addition, the ingestion of synbiotics (B. longum + inulin + oligofructose) significantly increases the abundance of bifidobacteria in the gut microbiota and butyrate production in the gut. However, to our knowledge, there are no reports on synbiotics increasing the adaptive immune response in the older adults for improving vaccine efficacy.
6.5. SCFAs
Table 4 lists dietary interventions that can improve COVID-19 vaccine efficacy by altering the gut microbiota in preclinical individuals. Adaptive immunity can be increased through SCFAs, which are the major metabolites of gut microbiota [[161], [162], [163]]. SCFAs are free fatty acids containing six carbons or less; the main SCFAs found in the intestine include acetate, propionate, and butyrate [164]. The ingestion of mixed SCFAs (acetate + butyrate + propionate) increases the antibody response to the influenza antigen in mice. The concentration of the three most abundant SCFAs (acetate + butyrate + propionate) was reduced in GF mice; this decreases the number of regulatory T cells. When GF mice ingest mixed SCFAs for 3 weeks, the number and function of intestinal regulatory T cells and the number of CD4+ T cells increase, contributing to the improvement of adaptive immunity. SCFAs increase the number of IgA antibody-producing B cells and CXCR5+ follicular T helper cells in the gut while simultaneously modulating antibody production by regulating the expression of key genes related to plasma cell differentiation. SCFA production in the gut of the older adults decreases owing to alterations in the composition of the gut microbiota [165], and the level of SCFAs is inversely related to age; the abundance of the genus Bifidobacterium decreases with age [166,167]. Therefore, the decrease in the adaptive immune response due to the reduction in SCFA production in the older adults could influence the decrease in vaccine efficacy; however, additional clinical studies are needed.
Table 4.
Dietary interventions to improve COVID-19 vaccine efficacy by modulating gut microbiota in preclinical subjects.
| Dietary interventions |
Study subject |
Intervention period |
Mechanism of improving the efficacy of COVID-19 vaccines |
References |
|---|---|---|---|---|
| Metabolites (SCFAs) | ||||
| · Mixed SCFAs: acetate (70 mM) + propionate (30 mM) + butyrate (20 mM) | · Mice | · 14 days | · Increased antibody production against influenza antigens | [161] |
| · Mixed SCFAs: acetate + propionate + butyrate (150 mM) | · Mice | · 3 weeks | · Improving adaptive immunity by increasing the number of CD4+ T cells in the gut | [162] |
| · Mixed SCFAs: acetate (70 mM) + propionate (30 mM) + butyrate (20 mM) | · Mice | · 4 weeks | · Increased numbers of IgA antibody-producing B cells and CXCR5+ follicular T helper cells in the gut | [163] |
| · Regulating antibody production by regulating the expression of key genes involved in plasma cell differentiation | ||||
Modulating the gut microbiota dysbiosis through dietary interventions should be considered for improving the efficacy of COVID-19 vaccines in the older adults. In addition, future studies should focus on identifying the most appropriate dietary interventions.
7. Conclusions and perspectives
COVID-19 started in Wuhan, China, in December 2019 and has spread worldwide, causing destruction in terms of the economy and the health and well-being of the population [168,169]. COVID-19 vaccination is one of the best methods to slow the spread of SARS-CoV-2 [170]. However, it is important to improve the efficacy of COVID-19 vaccines, as the efficacy is less in the older adults [171].
Dysbiosis of the gut microbiota occurs in the older adults [135], and this is positively correlated with the severity of COVID-19 in them [[121], [122], [123]]. A decrease in the efficacy of respiratory vaccines in the older adults is restored by modulating the gut microbiota [[103], [104], [105]]. This is achieved through simple dietary interventions, such as the ingestion of probiotics, next-generation probiotics, prebiotics, and synbiotics [59,[148], [149], [150], [151], [152], [153],[155], [156], [157],159,160].
Therefore, we propose the development of an efficient strategy for improving the reduced efficacy of COVID-19 vaccines in the older adults by modulating the gut microbiota through simple dietary interventions. However, more experimental studies are needed to prove the causal relationship between improved vaccine efficacy in the older adults and the modulation of gut microbiota through simple dietary interventions.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Data availability statement
No data was used for the research described in the article.
Additional information
No additional information is available for this paper.
Declaration of interest's statement
The authors declare no conflict of interest.
References
- 1.Wei M., Yang N., Wang F., Zhao G., Gao H., Li Y. Epidemiology of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Disaster Med. Public Health Prep. 2020;14:796–804. doi: 10.1017/dmp.2020.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pilishvili T., Gierke R., Fleming-Dutra K.E., Farrar J.L., Mohr N.M., Talan D.A., et al. Effectiveness of mRNA Covid-19 vaccine among US health care personnel. N. Engl. J. Med. 2021;385:e90. doi: 10.1056/NEJMoa2106599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bar-On Y.M., Goldberg Y., Mandel M., Bodenheimer O., Freedman L., Kalkstein N., et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N. Engl. J. Med. 2021;385:1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dagan N., Barda N., Kepten E., Miron O., Perchik S., Katz M.A., et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cevik M., Grubaugh N.D., Iwasaki A., Openshaw P. COVID-19 vaccines: keeping pace with SARS-CoV-2 variants. Cell. 2021;184:5077–5081. doi: 10.1016/j.cell.2021.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bubar K.M., Reinholt K., Kissler S.M., Lipsitch M., Cobey S., Grad Y.H., et al. Model-informed COVID-19 vaccine prioritization strategies by age and serostatus. Science. 2021;371:916–921. doi: 10.1126/science.abe6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinberger B. Adjuvant strategies to improve vaccination of the elderly population. Curr. Opin. Pharmacol. 2018;41:34–41. doi: 10.1016/j.coph.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 8.Reed S.G., Orr M.T., Fox C.B. Key roles of adjuvants in modern vaccines. Nat. Med. 2013;19:1597–1608. doi: 10.1038/nm.3409. [DOI] [PubMed] [Google Scholar]
- 9.Wong S.H., Lui R.N., Sung J.J. Covid‐19 and the digestive system. J. Gastroenterol. Hepatol. 2020;35:744–748. doi: 10.1111/jgh.15047. [DOI] [PubMed] [Google Scholar]
- 10.Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 11.Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831–1833. e3. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng D., Liwinski T., Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30:492–506. doi: 10.1038/s41422-020-0332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clemente J.C., Ursell L.K., Parfrey L.W., Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148:1258–1270. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gasmi A., Noor S., Tippairote T., Dadar M., Menzel A., Bjørklund G. Individual risk management strategy and potential therapeutic options for the COVID-19 pandemic. Clin. Immunol. 2020;215 doi: 10.1016/j.clim.2020.108409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dhar D., Mohanty A. Gut microbiota and Covid-19-possible link and implications. Virus Res. 2020;285 doi: 10.1016/j.virusres.2020.198018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zimmermann P., Curtis N. The influence of the intestinal microbiome on vaccine responses. Vaccine. 2018;36:4433–4439. doi: 10.1016/j.vaccine.2018.04.066. [DOI] [PubMed] [Google Scholar]
- 17.Lynn M.A., Tumes D.J., Choo J.M., Sribnaia A., Blake S.J., Leong L.E.X., et al. Early-life antibiotic-driven dysbiosis leads to dysregulated vaccine immune responses in mice. Cell Host Microbe. 2018;23:653–660. e5. doi: 10.1016/j.chom.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Huda M.N., Lewis Z., Kalanetra K.M., Rashid M., Ahmad S.M., Raqib R., et al. Stool microbiota and vaccine responses of infants. Pediatrics. 2014;134:e362–e372. doi: 10.1542/peds.2013-3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris V.C., Armah G., Fuentes S., Korpela K.E., Parashar U., Victor J.C., et al. Significant correlation between the infant gut microbiome and rotavirus vaccine response in rural Ghana. J. Infect. Dis. 2017;215:34–41. doi: 10.1093/infdis/jiw518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mojgani N., Shahali Y., Dadar M. Immune modulatory capacity of probiotic lactic acid bacteria and applications in vaccine development. Benef. Microbes. 2020;11:213–226. doi: 10.3920/BM2019.0121. [DOI] [PubMed] [Google Scholar]
- 21.Davidson L.E., Fiorino A.-M., Snydman D.R., Hibberd P.L. Lactobacillus GG as an immune adjuvant for live-attenuated influenza vaccine in healthy adults: a randomized double-blind placebo-controlled trial. Eur. J. Clin. Nutr. 2011;65:501–507. doi: 10.1038/ejcn.2010.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamilya D., Singh M. Springer; 2022. Probiotics as Vaccine Adjuvants. Probiotics in Aquaculture; pp. 203–212. [Google Scholar]
- 23.Peroni D.G., Morelli L. Probiotics as adjuvants in vaccine strategy: is there more room for improvement? Vaccines. 2021;9:811. doi: 10.3390/vaccines9080811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.A framework for human microbiome research. Nature. 2012;486:215–221. doi: 10.1038/nature11209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bagheri S.A.M. The University of Utah; 2018. Investigation of Microorganisms inside the Digestive Tracts. [Google Scholar]
- 26.Conlon M.A., Bird A.R. The impact of diet and lifestyle on gut microbiota and human health. Nutrients. 2014;7:17–44. doi: 10.3390/nu7010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beukema M., Faas M.M., de Vos P. The effects of different dietary fiber pectin structures on the gastrointestinal immune barrier: impact via gut microbiota and direct effects on immune cells. Exp. Mol. Med. 2020;52:1364–1376. doi: 10.1038/s12276-020-0449-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ciabattini A., Olivieri R., Lazzeri E., Medaglini D. Role of the microbiota in the modulation of vaccine immune responses. Front. Microbiol. 2019;10:1305. doi: 10.3389/fmicb.2019.01305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shroff K.E., Meslin K., Cebra J.J. Commensal enteric bacteria engender a self-limiting humoral mucosal immune response while permanently colonizing the gut. Infect. Immun. 1995;63:3904–3913. doi: 10.1128/iai.63.10.3904-3913.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.New J.S., Dizon B.L., Fucile C.F., Rosenberg A.F., Kearney J.F., King R.G. Neonatal exposure to commensal-bacteria-derived antigens directs polysaccharide-specific B-1 B cell repertoire development. Immunity. 2020;53:172–186. e6. doi: 10.1016/j.immuni.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li H., Limenitakis J.P., Greiff V., Yilmaz B., Schären O., Urbaniak C., et al. Mucosal or systemic microbiota exposures shape the B cell repertoire. Nature. 2020;584:274–278. doi: 10.1038/s41586-020-2564-6. [DOI] [PubMed] [Google Scholar]
- 32.Shi N., Li N., Duan X., Niu H. Interaction between the gut microbiome and mucosal immune system. Military Med Res. 2017;4:1–7. doi: 10.1186/s40779-017-0122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Torres J., Hu J., Seki A., Eisele C., Nair N., Huang R., et al. Infants born to mothers with IBD present with altered gut microbiome that transfers abnormalities of the adaptive immune system to germ-free mice. Gut. 2020;69:42–51. doi: 10.1136/gutjnl-2018-317855. [DOI] [PubMed] [Google Scholar]
- 34.Lynch S.V., Pedersen O. The human intestinal microbiome in health and disease. N. Engl. J. Med. 2016;375:2369–2379. doi: 10.1056/NEJMra1600266. [DOI] [PubMed] [Google Scholar]
- 35.Marchesi J.R., Adams D.H., Fava F., Hermes G.D., Hirschfield G.M., Hold G., et al. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65:330–339. doi: 10.1136/gutjnl-2015-309990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Y., Zhou J., Wang L. Role and mechanism of gut microbiota in human disease. Front. Cell. Infect. Microbiol. 2021:86. doi: 10.3389/fcimb.2021.625913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Odamaki T., Kato K., Sugahara H., Hashikura N., Takahashi S., Xiao J-z, et al. Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiol. 2016;16:1–12. doi: 10.1186/s12866-016-0708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fransen F., Van Beek A.A., Borghuis T., Aidy S.E., Hugenholtz F., van der Gaast–de Jongh C., et al. Aged gut microbiota contributes to systemical inflammaging after transfer to germ-free mice. Front. Immunol. 2017;8:1385. doi: 10.3389/fimmu.2017.01385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rinninella E., Raoul P., Cintoni M., Franceschi F., Miggiano G.A.D., Gasbarrini A., et al. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019;7:14. doi: 10.3390/microorganisms7010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hidalgo-Cantabrana C., Delgado S., Ruiz L., Ruas-Madiedo P., Sánchez B., Margolles A. Bugs as Drugs: Therapeutic Microbes for the Prevention and Treatment of Disease. 2018. Bifidobacteria and their health‐promoting effects; pp. 73–98. [Google Scholar]
- 41.Al-Sadi R., Dharmaprakash V., Nighot P., Guo S., Nighot M., Do T., et al. Bifidobacterium bifidum enhances the intestinal epithelial tight junction barrier and protects against intestinal inflammation by targeting the toll-like receptor-2 pathway in an nf-κb-independent manner. Int. J. Mol. Sci. 2021;22:8070. doi: 10.3390/ijms22158070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kurose Y., Minami J., Sen A., Iwabuchi N., Abe F., Xiao J., et al. Bioactive factors secreted by Bifidobacterium breve B-3 enhance barrier function in human intestinal Caco-2 cells. Benef. Microbes. 2019;10:89–100. doi: 10.3920/BM2018.0062. [DOI] [PubMed] [Google Scholar]
- 43.Mangin I., Dossou-Yovo F., Lévêque C., Dessoy M.-V., Sawoo O., Suau A., et al. Oral administration of viable Bifidobacterium pseudolongum strain Patronus modified colonic microbiota and increased mucus layer thickness in rat. FEMS Microbiol. Ecol. 2018;94:fiy177. doi: 10.1093/femsec/fiy177. [DOI] [PubMed] [Google Scholar]
- 44.Muhialdin B.J., Zawawi N., Razis A.F.A., Bakar J., Zarei M. Antiviral activity of fermented foods and their probiotics bacteria towards respiratory and alimentary tracts viruses. Food Control. 2021;127 doi: 10.1016/j.foodcont.2021.108140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gagnon M., Vimont A., Darveau A., Fliss I., Jean J. Study of the ability of bifidobacteria of human origin to prevent and treat rotavirus infection using colonic cell and mouse models. PLoS One. 2016;11 doi: 10.1371/journal.pone.0164512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ishizuka T., Kanmani P., Kobayashi H., Miyazaki A., Soma J., Suda Y., et al. Immunobiotic bifidobacteria strains modulate rotavirus immune response in porcine intestinal epitheliocytes via pattern recognition receptor signaling. PLoS One. 2016;11 doi: 10.1371/journal.pone.0152416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li D., Breiman A., Le Pendu J., Uyttendaele M. Anti-viral effect of Bifidobacterium adolescentis against noroviruses. Front. Microbiol. 2016;7:864. doi: 10.3389/fmicb.2016.00864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Botić T., Danø T., Weingartl H., Cencič A. A novel eukaryotic cell culture model to study antiviral activity of potential probiotic bacteria. Int. J. Food Microbiol. 2007;115:227–234. doi: 10.1016/j.ijfoodmicro.2006.10.044. [DOI] [PubMed] [Google Scholar]
- 49.Butcher S., Chahel H., Lord J. Ageing and the neutrophil: no appetite for killing? Immunology. 2000;100:411–416. doi: 10.1046/j.1365-2567.2000.00079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gill H.S., Rutherfurd K.J., Cross M.L., Gopal P.K. Enhancement of immunity in the elderly by dietary supplementation with the probiotic Bifidobacterium lactis HN019. Am. J. Clin. Nutr. 2001;74:833–839. doi: 10.1093/ajcn/74.6.833. [DOI] [PubMed] [Google Scholar]
- 51.Maneerat S., Lehtinen M.J., Childs C.E., Forssten S.D., Alhoniemi E., Tiphaine M., et al. Consumption of Bifidobacterium lactis Bi-07 by healthy elderly adults enhances phagocytic activity of monocytes and granulocytes. J. Nutr. Sci. 2013;2 doi: 10.1017/jns.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Namba K., Hatano M., Yaeshima T., Takase M., Suzuki K. Effects of Bifidobacterium longum BB536 administration on influenza infection, influenza vaccine antibody titer, and cell-mediated immunity in the elderly. Biosci. Biotechnol. Biochem. 2010;74:939–945. doi: 10.1271/bbb.90749. [DOI] [PubMed] [Google Scholar]
- 53.El-Bakry H.A., Zahran W.M., Anter S.A., Zahran A.S. Role of some selected Bifidobacterium strains in modulating immunosenescence of aged albino rats. J Basic Appl Zool. 2013;66:255–262. [Google Scholar]
- 54.You J., Yaqoob P. Evidence of immunomodulatory effects of a novel probiotic, Bifidobacterium longum bv. infantis CCUG 52486. FEMS Immunol. Med. Microbiol. 2012;66:353–362. doi: 10.1111/j.1574-695X.2012.01014.x. [DOI] [PubMed] [Google Scholar]
- 55.Wu B.-B., Yang Y., Xu X., Wang W.-P. Effects of Bifidobacterium supplementation on intestinal microbiota composition and the immune response in healthy infants. World J Pediatr. 2016;12:177–182. doi: 10.1007/s12519-015-0025-3. [DOI] [PubMed] [Google Scholar]
- 56.Jeon S.G., Kayama H., Ueda Y., Takahashi T., Asahara T., Tsuji H., et al. Probiotic Bifidobacterium breve induces IL-10-producing Tr1 cells in the colon. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1002714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lyons A., O'mahony D., O'brien F., MacSharry J., Sheil B., Ceddia M., et al. Bacterial strain‐specific induction of Foxp3+ T regulatory cells is protective in murine allergy models. Clin. Exp. Allergy. 2010;40:811–819. doi: 10.1111/j.1365-2222.2009.03437.x. [DOI] [PubMed] [Google Scholar]
- 58.Verma R., Lee C., Jeun E.-J., Yi J., Kim K.S., Ghosh A., et al. Cell surface polysaccharides of Bifidobacterium bifidum induce the generation of Foxp3+ regulatory T cells. Sci Immunol. 2018;3 doi: 10.1126/sciimmunol.aat6975. [DOI] [PubMed] [Google Scholar]
- 59.Akatsu H., Iwabuchi N., Xiao Jz, Matsuyama Z., Kurihara R., Okuda K., et al. Clinical effects of probiotic Bifidobacterium longum BB536 on immune function and intestinal microbiota in elderly patients receiving enteral tube feeding. J. Parenter. Enteral Nutr. 2013;37:631–640. doi: 10.1177/0148607112467819. [DOI] [PubMed] [Google Scholar]
- 60.Rodriguez-Zhurbenko N., Quach T.D., Hopkins T.J., Rothstein T.L., Hernandez A.M. Human B-1 cells and B-1 cell antibodies change with advancing age. Front. Immunol. 2019;10:483. doi: 10.3389/fimmu.2019.00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Davinelli S., Scapagnini G. Interactions between dietary polyphenols and aging gut microbiota: a review. Biofactors. 2022;48:274–284. doi: 10.1002/biof.1785. [DOI] [PubMed] [Google Scholar]
- 62.Sánchez-Campos S., Fernández M.J., Porras D., García-Mediavilla M.V., Román-Sagüillo S., González-Gallego J., et al. 2008. Gut Microbiota in the Elderly. [Google Scholar]
- 63.Biragyn A., Ferrucci L. Gut dysbiosis: a potential link between increased cancer risk in ageing and inflammaging. Lancet Oncol. 2018;19:e295–e304. doi: 10.1016/S1470-2045(18)30095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.He Y., Wen Q., Yao F., Xu D., Huang Y., Wang J. Gut–lung axis: the microbial contributions and clinical implications. Crit. Rev. Microbiol. 2017;43:81–95. doi: 10.1080/1040841X.2016.1176988. [DOI] [PubMed] [Google Scholar]
- 65.Roussos A., Koursarakos P., Patsopoulos D., Gerogianni I., Philippou N. Increased prevalence of irritable bowel syndrome in patients with bronchial asthma. Respir. Med. 2003;97:75–79. doi: 10.1053/rmed.2001.1409. [DOI] [PubMed] [Google Scholar]
- 66.Lee J., Im J.P., Han K., Park S., Soh H., Choi K., et al. Risk of inflammatory bowel disease in patients with chronic obstructive pulmonary disease: a nationwide, population-based study. World J. Gastroenterol. 2019;25:6354. doi: 10.3748/wjg.v25.i42.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dilantika C., Sedyaningsih E.R., Kasper M.R., Agtini M., Listiyaningsih E., Uyeki T.M., et al. Influenza virus infection among pediatric patients reporting diarrhea and influenza-like illness. BMC Infect. Dis. 2010;10:1–5. doi: 10.1186/1471-2334-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dominguez S.R., Robinson C.C., Holmes K.V. Detection of four human coronaviruses in respiratory infections in children: a one‐year study in Colorado. J. Med. Virol. 2009;81:1597–1604. doi: 10.1002/jmv.21541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yazar A., Atis S., Konca K., Pata C., Akbay E., Calikoglu M., et al. Respiratory symptoms and pulmonary functional changes in patients with irritable bowel syndrome. Am. J. Gastroenterol. 2001;96:1511–1516. doi: 10.1111/j.1572-0241.2001.03748.x. [DOI] [PubMed] [Google Scholar]
- 70.Lai H.-C., Lin H.-J., Kao Y.-W., Wang K.-H., Chou J.-W., Shia B.-C., et al. Irritable bowel syndrome increases the risk of chronic obstructive pulmonary disease: a retrospective cohort study. Sci. Rep. 2020;10:1–11. doi: 10.1038/s41598-020-66707-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bingula R., Filaire M., Radosevic-Robin N., Bey M., Berthon J.-Y., Bernalier-Donadille A., et al. Desired turbulence? Gut-lung axis, immunity, and lung cancer. JAMA Oncol. 2017;2017 doi: 10.1155/2017/5035371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wedgwood S., Warford C., Agvatisiri S.R., Thai P.N., Chiamvimonvat N., Kalanetra K.M., et al. The developing gut–lung axis: postnatal growth restriction, intestinal dysbiosis, and pulmonary hypertension in a rodent model. Pediatr. Res. 2020;87:472–479. doi: 10.1038/s41390-019-0578-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shreiner A.B., Kao J.Y., Young V.B. The gut microbiome in health and in disease. Curr. Opin. Gastroenterol. 2015;31:69. doi: 10.1097/MOG.0000000000000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Daley D. The evolution of the hygiene hypothesis: the role of early-life exposures to viruses and microbes and their relationship to asthma and allergic diseases. Curr. Opin. Allergy Clin. Immunol. 2014;14:390–396. doi: 10.1097/ACI.0000000000000101. [DOI] [PubMed] [Google Scholar]
- 75.Wang Q., Li F., Liang B., Liang Y., Chen S., Mo X., et al. A metagenome-wide association study of gut microbiota in asthma in UK adults. BMC Microbiol. 2018;18:1–7. doi: 10.1186/s12866-018-1257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abrahamsson T., Jakobsson H., Andersson A.F., Björkstén B., Engstrand L., Jenmalm M. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin. Exp. Allergy. 2014;44:842–850. doi: 10.1111/cea.12253. [DOI] [PubMed] [Google Scholar]
- 77.Fujimura K.E., Sitarik A.R., Havstad S., Lin D.L., Levan S., Fadrosh D., et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat. Med. 2016;22:1187–1191. doi: 10.1038/nm.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bowerman K.L., Rehman S.F., Vaughan A., Lachner N., Budden K.F., Kim R.Y., et al. Disease-associated gut microbiome and metabolome changes in patients with chronic obstructive pulmonary disease. Nat. Commun. 2020;11:1–15. doi: 10.1038/s41467-020-19701-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lai H.-C., Lin T.-L., Chen T.-W., Kuo Y.-L., Chang C.-J., Wu T.-R., et al. Gut microbiota modulates COPD pathogenesis: role of anti-inflammatory Parabacteroides goldsteinii lipopolysaccharide. Gut. 2022;71:309–321. doi: 10.1136/gutjnl-2020-322599. [DOI] [PubMed] [Google Scholar]
- 80.Tam R.Y., van Dorst J.M., McKay I., Coffey M., Ooi C.Y. Intestinal inflammation and alterations in the gut microbiota in cystic fibrosis: a review of the current evidence, pathophysiology and future directions. J. Clin. Med. 2022;11:649. doi: 10.3390/jcm11030649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Saint-Criq V., Lugo-Villarino G., Thomas M. Dysbiosis, malnutrition and enhanced gut-lung axis contribute to age-related respiratory diseases. Ageing Res. Rev. 2021;66 doi: 10.1016/j.arr.2020.101235. [DOI] [PubMed] [Google Scholar]
- 82.Meyer K.C. Seminars in Respiratory and Critical Care Medicine. © Thieme Medical Publishers; 2010. The role of immunity and inflammation in lung senescence and susceptibility to infection in the elderly; pp. 561–574. [DOI] [PubMed] [Google Scholar]
- 83.Vital M., Harkema J.R., Rizzo M., Tiedje J., Brandenberger C. Alterations of the murine gut microbiome with age and allergic airway disease. J Immunol Res. 2015;2015 doi: 10.1155/2015/892568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim Y.-G. Microbiota influences vaccine and mucosal adjuvant efficacy. Immun Netw. 2017;17:20–24. doi: 10.4110/in.2017.17.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gustafson C.E., Kim C., Weyand C.M., Goronzy J.J. Influence of immune aging on vaccine responses. J. Allergy Clin. Immunol. 2020;145:1309–1321. doi: 10.1016/j.jaci.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fischinger S., Boudreau C.M., Butler A.L., Streeck H., Alter G. Seminars in Immunopathology. Springer; 2019. Sex differences in vaccine-induced humoral immunity; pp. 239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kimman T., Vandebriel R., Hoebee B. Genetic variation in the response to vaccination. Public Health Genomics. 2007;10:201–217. doi: 10.1159/000106559. [DOI] [PubMed] [Google Scholar]
- 88.Bell M.R., Kutzler M.A. An old problem with new solutions: strategies to improve vaccine efficacy in the elderly. Adv. Drug Deliv. Rev. 2022 doi: 10.1016/j.addr.2022.114175. [DOI] [PubMed] [Google Scholar]
- 89.Osterholm M.T., Kelley N.S., Sommer A., BelonJ Allergy Clin Immunolgia E.A. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect. Dis. 2012;12:36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- 90.Goodwin K., Viboud C., Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006;24:1159–1169. doi: 10.1016/j.vaccine.2005.08.105. [DOI] [PubMed] [Google Scholar]
- 91.Zhao J., Zhao J., Legge K., Perlman S. Age-related increases in PGD 2 expression impair respiratory DC migration, resulting in diminished T cell responses upon respiratory virus infection in mice. J. Clin. Invest. 2011;121:4921–4930. doi: 10.1172/JCI59777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Leroux-Roels G. Unmet needs in modern vaccinology: adjuvants to improve the immune response. Vaccine. 2010;28:C25–C36. doi: 10.1016/j.vaccine.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 93.Baylor N.W., Egan W., Richman P. Aluminum salts in vaccines—US perspective. Vaccine. 2002;20:S18–S23. doi: 10.1016/s0264-410x(02)00166-4. [DOI] [PubMed] [Google Scholar]
- 94.Huckriede A., Bungener L., Stegmann T., Daemen T., Medema J., Palache A.M., et al. The virosome concept for influenza vaccines. Vaccine. 2005;23:S26–S38. doi: 10.1016/j.vaccine.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 95.Lin Y.-J., Wen C.-N., Lin Y.-Y., Hsieh W.-C., Chang C.-C., Chen Y.-H., et al. Oil-in-water emulsion adjuvants for pediatric influenza vaccines: a systematic review and meta-analysis. Nat. Commun. 2020;11:1–12. doi: 10.1038/s41467-019-14230-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tomljenovic L., Shaw A. Aluminum vaccine adjuvants: are they safe? Curr. Med. Chem. 2011;18:2630–2637. doi: 10.2174/092986711795933740. [DOI] [PubMed] [Google Scholar]
- 97.Shaw C., Tomljenovic L. Aluminum in the central nervous system (CNS): toxicity in humans and animals, vaccine adjuvants, and autoimmunity. Immunol. Res. 2013;56:304–316. doi: 10.1007/s12026-013-8403-1. [DOI] [PubMed] [Google Scholar]
- 98.Tomljenovic L., Shaw C.A. Do aluminum vaccine adjuvants contribute to the rising prevalence of autism? J. Inorg. Biochem. 2011;105:1489–1499. doi: 10.1016/j.jinorgbio.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 99.Oh J.Z., Ravindran R., Chassaing B., Carvalho F.A., Maddur M.S., Bower M., et al. TLR5-mediated sensing of gut microbiota is necessary for antibody responses to seasonal influenza vaccination. Immunity. 2014;41:478–492. doi: 10.1016/j.immuni.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Youngster I., Kozer E., Lazarovitch Z., Broide E., Goldman M. Probiotics and the immunological response to infant vaccinations: a prospective, placebo controlled pilot study. Arch. Dis. Child. 2011;96:345–349. doi: 10.1136/adc.2010.197459. [DOI] [PubMed] [Google Scholar]
- 101.West C.E., Gothefors L., Granström M., Käyhty H., Hammarström M.L.K., Hernell O. Effects of feeding probiotics during weaning on infections and antibody responses to diphtheria, tetanus and Hib vaccines. Pediatr. Allergy Immunol. 2008;19:53–60. doi: 10.1111/j.1399-3038.2007.00583.x. [DOI] [PubMed] [Google Scholar]
- 102.Olivares M., Díaz-Ropero M.P., Sierra S., Lara-Villoslada F., Fonollá J., Navas M., et al. Oral intake of Lactobacillus fermentum CECT5716 enhances the effects of influenza vaccination. Nutrition. 2007;23:254–260. doi: 10.1016/j.nut.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 103.Boge T., Rémigy M., Vaudaine S., Tanguy J., Bourdet-Sicard R., van der Werf S. A probiotic fermented dairy drink improves antibody response to influenza vaccination in the elderly in two randomised controlled trials. Vaccine. 2009;27:5677–5684. doi: 10.1016/j.vaccine.2009.06.094. [DOI] [PubMed] [Google Scholar]
- 104.Bosch M., Mendez M., Perez M., Farran A., Fuentes M., Cune J. Lactobacillus plantarum CECT7315 and CECT7316 stimulate immunoglobulin production after influenza vaccination in elderly. Nutr. Hosp. 2012;27:504–509. doi: 10.1590/S0212-16112012000200023. [DOI] [PubMed] [Google Scholar]
- 105.Sandionigi A., De Giani A., Tursi F., Michelotti A., Cestone E., Giardina S., et al. Effectiveness of multistrain probiotic formulation on common infectious disease symptoms and gut microbiota modulation in flu-vaccinated healthy elderly subjects. BioMed Res. Int. 2022:2022. doi: 10.1155/2022/3860896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wong M.C., Huang J., Lai C., Ng R., Chan F.K., Chan P.K. Detection of SARS-CoV-2 RNA in fecal specimens of patients with confirmed COVID-19: a meta-analysis. J. Infect. 2020;81:e31–e38. doi: 10.1016/j.jinf.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.D’amico F., Baumgart D.C., Danese S., Peyrin-Biroulet L. Diarrhea during COVID-19 infection: pathogenesis, epidemiology, prevention, and management. Clin. Gastroenterol. Hepatol. 2020;18:1663–1672. doi: 10.1016/j.cgh.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gu J., Han B., Wang J. COVID-19: gastrointestinal manifestations and potential fecal–oral transmission. Gastroenterology. 2020;158:1518–1519. doi: 10.1053/j.gastro.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Guney C., Akar F. Epithelial and endothelial expressions of ACE2: SARS-CoV-2 entry routes. J. Pharm. Pharmaceut. Sci. 2021;24:84–93. doi: 10.18433/jpps31455. [DOI] [PubMed] [Google Scholar]
- 111.Li M.-Y., Li L., Zhang Y., Wang X.-S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9:23–29. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lee J.J., Kopetz S., Vilar E., Shen J.P., Chen K., Maitra A. Relative abundance of SARS-CoV-2 entry genes in the enterocytes of the lower gastrointestinal tract. Genes. 2020;11:645. doi: 10.3390/genes11060645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wan S., Xiang Y., Fang W., Zheng Y., Li B., Hu Y., et al. Clinical features and treatment of COVID‐19 patients in northeast Chongqing. J. Med. Virol. 2020;92:797–806. doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Alimohamadi Y., Sepandi M., Taghdir M., Hosamirudsari H. Determine the most common clinical symptoms in COVID-19 patients: a systematic review and meta-analysis. J Prev Med Hyg. 2020;61:E304. doi: 10.15167/2421-4248/jpmh2020.61.3.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pan L., Mu M., Yang P., Sun Y., Wang R., Yan J., et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am. J. Gastroenterol. 2020;115 doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lin L., Jiang X., Zhang Z., Huang S., Zhang Z., Fang Z., et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69:997–1001. doi: 10.1136/gutjnl-2020-321013. [DOI] [PubMed] [Google Scholar]
- 117.Cheung K.S., Hung I.F., Chan P.P., Lung K., Tso E., Liu R., et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a Hong Kong cohort: systematic review and meta-analysis. Gastroenterology. 2020;159:81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zeng W., Qi K., Ye M., Zheng L., Liu X., Hu S., et al. Gastrointestinal symptoms are associated with severity of coronavirus disease 2019: a systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2022;34:168–176. doi: 10.1097/MEG.0000000000002072. [DOI] [PubMed] [Google Scholar]
- 119.Ren Z., Wang H., Cui G., Lu H., Wang L., Luo H., et al. Alterations in the human oral and gut microbiomes and lipidomics in COVID-19. Gut. 2021;70:1253–1265. doi: 10.1136/gutjnl-2020-323826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gu S., Chen Y., Wu Z., Chen Y., Gao H., Lv L., et al. Alterations of the gut microbiota in patients with coronavirus disease 2019 or H1N1 influenza. Clin. Infect. Dis. 2020;71:2669–2678. doi: 10.1093/cid/ciaa709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yeoh Y.K., Zuo T., Lui G.C.-Y., Zhang F., Liu Q., Li A.Y., et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021;70:698–706. doi: 10.1136/gutjnl-2020-323020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zuo T., Zhang F., Lui G.C., Yeoh Y.K., Li A.Y., Zhan H., et al. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology. 2020;159:944–955. e8. doi: 10.1053/j.gastro.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zuo T., Liu Q., Zhang F., Lui G.C.-Y., Tso E.Y., Yeoh Y.K., et al. Depicting SARS-CoV-2 faecal viral activity in association with gut microbiota composition in patients with COVID-19. Gut. 2021;70:276–284. doi: 10.1136/gutjnl-2020-322294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Valdes A.M., Walter J., Segal E., Spector T.D. Role of the gut microbiota in nutrition and health. BMJ. 2018:361. doi: 10.1136/bmj.k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Haak B.W., Littmann E.R., Chaubard J.-L., Pickard A.J., Fontana E., Adhi F., et al. Impact of gut colonization with butyrate-producing microbiota on respiratory viral infection following allo-HCT. Blood. 2018;131:2978–2986. doi: 10.1182/blood-2018-01-828996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen J., Vitetta L. The role of butyrate in attenuating pathobiont-induced hyperinflammation. Immun Netw. 2020;20 doi: 10.4110/in.2020.20.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chen J., Hall S., Vitetta L. Altered gut microbial metabolites could mediate the effects of risk factors in Covid‐19. Rev. Med. Virol. 2021;31:1–13. doi: 10.1002/rmv.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kulkarni P., Devkumar P., Chattopadhyay I. Could dysbiosis of inflammatory and anti-inflammatory gut bacteria have an implications in the development of type 2 diabetes? A pilot investigation. BMC Res. Notes. 2021;14:1–7. doi: 10.1186/s13104-021-05466-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Murphy K., Ryan C., Dempsey E.M., O'Toole P.W., Ross R.P., Stanton C., et al. Neonatal sulfhemoglobinemia and hemolytic anemia associated with intestinal Morganella morganii. Pediatrics. 2015;136:e1641–e1645. doi: 10.1542/peds.2015-0996. [DOI] [PubMed] [Google Scholar]
- 130.Smorenberg A., Peters E.J., van Daele P.L., Nossent E.J., Muller M. How does SARS-CoV-2 targets the elderly patients? A review on potential mechanisms increasing disease severity. Eur. J. Intern. Med. 2021;83:1–5. doi: 10.1016/j.ejim.2020.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wan Y., Li J., Shen L., Zou Y., Hou L., Zhu L., et al. Enteric involvement in hospitalised patients with COVID-19 outside Wuhan. Lancet Gastroenterol Hepatol. 2020;5:534–535. doi: 10.1016/S2468-1253(20)30118-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wu J., Li J., Zhu G., Zhang Y., Bi Z., Yu Y., et al. Clinical features of maintenance hemodialysis patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. Clin. J. Am. Soc. Nephrol. 2020;15:1139–1145. doi: 10.2215/CJN.04160320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xu C., Zhu H., Qiu P. Aging progression of human gut microbiota. BMC Microbiol. 2019;19:1–10. doi: 10.1186/s12866-019-1616-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Doherty J., Fennessy S., Stack R., O'Morain N., Cullen G., Ryan E.J., et al. Vaccination for patients with inflammatory bowel disease during the COVID‐19 pandemic. Aliment. Pharmacol. Ther. 2021;54:1110–1123. doi: 10.1111/apt.16590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kappelman M.D., Weaver K.N., Boccieri M., Firestine A., Zhang X., Long M.D., et al. Humoral immune response to messenger RNA COVID-19 vaccines among patients with inflammatory bowel disease. Gastroenterology. 2021;161:1340–1343. e2. doi: 10.1053/j.gastro.2021.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ng S.C., Peng Y., Zhang L., Mok C.K., Zhao S., Li A., et al. Gut microbiota composition is associated with SARS-CoV-2 vaccine immunogenicity and adverse events. Gut. 2022;71:1106–1116. doi: 10.1136/gutjnl-2021-326563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Vassilaki N., Gargalionis A.N., Bletsa A., Papamichalopoulos N., Kontou E., Gkika M., et al. Impact of age and sex on antibody response following the second dose of COVID-19 BNT162b2 mRNA vaccine in Greek healthcare workers. Microorganisms. 2021;9:1725. doi: 10.3390/microorganisms9081725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Müller L., Andrée M., Moskorz W., Drexler I., Walotka L., Grothmann R., et al. Age-dependent immune response to the Biontech/Pfizer BNT162b2 coronavirus disease 2019 vaccination. Clin. Infect. Dis. 2021;73:2065–2072. doi: 10.1093/cid/ciab381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Arregocés-Castillo L., Fernández-Niño J., Rojas-Botero M., Palacios-Clavijo A., Galvis-Pedraza M., Rincón-Medrano L., et al. Effectiveness of COVID-19 vaccines in older adults in Colombia: a retrospective, population-based study of the ESPERANZA cohort. Lancet Healthy Longev. 2022;3:e242–e252. doi: 10.1016/S2666-7568(22)00035-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nelde A., Bilich T., Heitmann J.S., Maringer Y., Salih H.R., Roerden M., et al. SARS-CoV-2-derived peptides define heterologous and COVID-19-induced T cell recognition. Nat. Immunol. 2021;22:74–85. doi: 10.1038/s41590-020-00808-x. [DOI] [PubMed] [Google Scholar]
- 143.Wack A., Cossarizza A., Heltai S., Barbieri D., D'Addato S., Fransceschi C., et al. Age-related modifications of the human alphabeta T cell repertoire due to different clonal expansions in the CD4+ and CD8+ subsets. Int. Immunol. 1998;10:1281–1288. doi: 10.1093/intimm/10.9.1281. [DOI] [PubMed] [Google Scholar]
- 144.Britanova O.V., Putintseva E.V., Shugay M., Merzlyak E.M., Turchaninova M.A., Staroverov D.B., et al. Age-related decrease in TCR repertoire diversity measured with deep and normalized sequence profiling. J. Immunol. 2014;192:2689–2698. doi: 10.4049/jimmunol.1302064. [DOI] [PubMed] [Google Scholar]
- 145.Thomas R., Wang W., Su D.-M. Contributions of age-related thymic involution to immunosenescence and inflammaging. Immun. Ageing. 2020;17:1–17. doi: 10.1186/s12979-020-0173-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Huang I., Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. J Intens Care. 2020;8:1–10. doi: 10.1186/s40560-020-00453-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fagnoni F.F., Vescovini R., Passeri G., Bologna G., Pedrazzoni M., Lavagetto G., et al. Shortage of circulating naive CD8+ T cells provides new insights on immunodeficiency in aging. Blood. 2000;95:2860–2868. [PubMed] [Google Scholar]
- 148.Finamore A., Roselli M., Donini L., Brasili E., Rami R., Carnevali P., et al. Supplementation with Bifidobacterium longum Bar33 and Lactobacillus helveticus Bar13 mixture improves immunity in elderly humans (over 75 years) and aged mice. Nutrition. 2019;63:184–192. doi: 10.1016/j.nut.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 149.Wang L., Zhang J., Guo Z., Kwok L., Ma C., Zhang W., et al. Effect of oral consumption of probiotic Lactobacillus planatarum P-8 on fecal microbiota, SIgA, SCFAs, and TBAs of adults of different ages. Nutrition. 2014;30:776–783. e1. doi: 10.1016/j.nut.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 150.Maruyama M., Abe R., Shimono T., Iwabuchi N., Abe F., Xiao J.-Z. The effects of non-viable Lactobacillus on immune function in the elderly: a randomised, double-blind, placebo-controlled study. Int. J. Food Sci. Nutr. 2016;67:67–73. doi: 10.3109/09637486.2015.1126564. [DOI] [PubMed] [Google Scholar]
- 151.Moro-García M.A., Alonso-Arias R., Baltadjieva M., Fernández Benítez C., Fernández Barrial M.A., Díaz Ruisánchez E., et al. Oral supplementation with Lactobacillus delbrueckii subsp. bulgaricus 8481 enhances systemic immunity in elderly subjects. Age. 2013;35:1311–1326. doi: 10.1007/s11357-012-9434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lin T.-L., Shu C.-C., Lai W.-F., Tzeng C.-M., Lai H.-C., Lu C.-C. Investiture of next generation probiotics on amelioration of diseases–Strains do matter. Med Microecol. 2019;1 [Google Scholar]
- 153.He Q., Hou Q., Wang Y., Shen L., Sun Z., Zhang H., et al. Long-term administration of Lactobacillus casei Zhang stabilized gut microbiota of adults and reduced gut microbiota age index of older adults. J. Funct.Foods. 2020;64 [Google Scholar]
- 154.Slavin J. Fiber and prebiotics: mechanisms and health benefits. Nutrients. 2013;5:1417–1435. doi: 10.3390/nu5041417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Vulevic J., Drakoularakou A., Yaqoob P., Tzortzis G., Gibson G.R. Modulation of the fecal microflora profile and immune function by a novel trans-galactooligosaccharide mixture (B-GOS) in healthy elderly volunteers. Am. J. Clin. Nutr. 2008;88:1438–1446. doi: 10.3945/ajcn.2008.26242. [DOI] [PubMed] [Google Scholar]
- 156.Simpson H.L., Campbell B.J. Dietary fibre–microbiota interactions. Aliment. Pharmacol. Ther. 2015;42:158–179. doi: 10.1111/apt.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nagafuchi S., Yamaji T., Kawashima A., Saito Y., Takahashi T., Yamamoto T., et al. Effects of a formula containing two types of prebiotics, bifidogenic growth stimulator and galacto-oligosaccharide, and fermented milk products on intestinal microbiota and antibody response to influenza vaccine in elderly patients: a randomized controlled trial. Pharmaceuticals. 2015;8:351–365. doi: 10.3390/ph8020351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Markowiak P., Śliżewska K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients. 2017;9:1021. doi: 10.3390/nu9091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bartosch S., Woodmansey E.J., Paterson J.C., McMurdo M.E., Macfarlane G.T. Microbiological effects of consuming a synbiotic containing Bifidobacterium bifidum, Bifidobacterium lactis, and oligofructose in elderly persons, determined by real-time polymerase chain reaction and counting of viable bacteria. Clin. Infect. Dis. 2005;40:28–37. doi: 10.1086/426027. [DOI] [PubMed] [Google Scholar]
- 160.Macfarlane S., Cleary S., Bahrami B., Reynolds N., Macfarlane G. Synbiotic consumption changes the metabolism and composition of the gut microbiota in older people and modifies inflammatory processes: a randomised, double‐blind, placebo‐controlled crossover study. Aliment. Pharmacol. Ther. 2013;38:804–816. doi: 10.1111/apt.12453. [DOI] [PubMed] [Google Scholar]
- 161.Cait A., Mooney A., Poyntz H., Shortt N., Jones A., Gestin A., et al. Potential association between dietary fibre and humoral response to the seasonal influenza vaccine. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.765528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Smith P.M., Howitt M.R., Panikov N., Michaud M., Gallini C.A., Bohlooly-y M., et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kim M., Qie Y., Park J., Kim C.H. Gut microbial metabolites fuel host antibody responses. Cell Host Microbe. 2016;20:202–214. doi: 10.1016/j.chom.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Macfarlane S., Macfarlane G.T. Regulation of short-chain fatty acid production. Proc. Nutr. Soc. 2003;62:67–72. doi: 10.1079/PNS2002207. [DOI] [PubMed] [Google Scholar]
- 165.Vemuri R., Gundamaraju R., Shastri M.D., Shukla S.D., Kalpurath K., Ball M., et al. Gut microbial changes, interactions, and their implications on human lifecycle: an ageing perspective. BioMed Res. Int. 2018;2018 doi: 10.1155/2018/4178607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Salazar N., López P., Valdés L., Margolles A., Suárez A., Patterson A.M., et al. Microbial targets for the development of functional foods accordingly with nutritional and immune parameters altered in the elderly. J. Am. Coll. Nutr. 2013;32:399–406. doi: 10.1080/07315724.2013.827047. [DOI] [PubMed] [Google Scholar]
- 167.Salazar N., Arboleya S., Fernández-Navarro T., de Los Reyes-Gavilán C.G., Gonzalez S., Gueimonde M. Age-associated changes in gut microbiota and dietary components related with the immune system in adulthood and old age: a cross-sectional study. Nutrients. 2019;11:1765. doi: 10.3390/nu11081765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kumar A., Singh R., Kaur J., Pandey S., Sharma V., Thakur L., et al. Wuhan to world: the COVID-19 pandemic. Front. Cell. Infect. Microbiol. 2021:242. doi: 10.3389/fcimb.2021.596201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.England N. HM Government; London: 2020. Analysis of the Health, Economic and Social Effects of Covid-19 and the Approach to Tiering. [Google Scholar]
- 170.Organization W.H. 2022. Statement for Healthcare Professionals: How COVID-19 Vaccines Are Regulated for Safety and Effectiveness (Revised March 2022) [Google Scholar]
- 171.Soiza R.L., Scicluna C., Thomson E.C. Efficacy and safety of COVID-19 vaccines in older people. Age Ageing. 2021;50:279–283. doi: 10.1093/ageing/afaa274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rizzardini G., Eskesen D., Calder P.C., Capetti A., Jespersen L., Clerici M. Evaluation of the immune benefits of two probiotic strains Bifidobacterium animalis ssp. lactis, BB-12® and Lactobacillus paracasei ssp. paracasei, L. casei 431® in an influenza vaccination model: a randomised, double-blind, placebo-controlled study. Br. J. Nutr. 2012;107:876–884. doi: 10.1017/S000711451100420X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.