Abstract
Background
Cognitive effects of tamoxifen have been described. We augment data from a previous short-term (ST) follow-up study with long-term (LT) data to evaluate ST and LT cognitive effects of tamoxifen followed by exemestane and exemestane in breast cancer patients.
Methods
Patients from the Tamoxifen and Exemestane Adjuvant Multinational trial received 5 years exemestane (exemestane group, n = 114) or 2.5 years tamoxifen followed by 2.5 years exemestane (sequential group, n = 92). Neuropsychological performance was assessed pre-endocrine therapy, after 1 year (ST follow-up) and at 5 years (LT follow-up). A control group of healthy participants (n = 120) were assessed with parallel intervals. With random effects modeling we evaluated cognitive changes from baseline to ST and LT follow-up. Statistical tests were 2-sided.
Results
After controlling for age, intelligence quotient, attrition, menopausal symptoms, anxiety and/or depression, and/or fatigue, the sequential group showed ST and LT decline compared with control participants on verbal memory (effect size [ES] = 0.26, P = .01; ES = 0.34, P = .003) and executive function (ES = 0.27, P = .007; ES = 0.38, P = .002). Compared with the exemestane group, the sequential group demonstrated ST decline on information processing speed (ES = 0.33, P = .01) and executive function (ES = 0.32, P = .01) and LT decline on verbal memory (ES = 0.33, P = .02). The exemestane group showed no cognitive decline compared with control participants.
Conclusion
Cognitive adverse effects of tamoxifen alone and after switching to exemestane were observed, suggestive of a carryover effect of tamoxifen. Our results underline the need for well-controlled, prospective trials studying cognitive effects of endocrine therapy.
Adjuvant endocrine therapy (ET) is standard of care in the treatment of hormone receptor-positive breast cancer (BC). Commonly, 2 types of ET are given, depending largely on the patient’s menopausal status. Selective estrogen receptor (ER) modulators (eg, tamoxifen) block ERs on BC cells, and aromatase inhibitors (AIs; eg, anastrozole, exemestane, and letrozole) inhibit production of estrogens by inactivating the enzyme aromatase in peripheral adipose tissue. Premenopausal women often receive tamoxifen with ovarian function suppression or an AI with ovarian function suppression for 5-10 years postsurgery. In postmenopausal women, common treatments include 5 years of AI, 5 years of tamoxifen followed by an AI for 2-3 years, or tamoxifen for 2-3 years followed by an AI up to 5 years (1).
ET may come with side effects. Cognitive problems are frequently reported symptoms in BC patients using ET. The brain is widely responsive to estrogens. Important areas for cognition such as the hippocampus and frontal lobes are sensitive to estrogens. Therefore, downregulation of estrogen production or blocking its activity through ET could impact cognition (2-4).
Several observational studies and randomized controlled trials using cognitive tests indicate that cognitive adverse effects of ET may exist and may differ between ET agents (5-9). A recent comprehensive review reported frequency rates of cognitive dysfunction in 32% to 64% of patients receiving ET, with conflicting results on the differential impact of ET types (10,11). Unfortunately, many studies had limitations including small sample sizes, short observation period, heterogeneity of ET and duration of use, and interference of other potentially neurotoxic therapies such as chemotherapy. Also, few studies have directly examined differences in cognitive effects between ET agents using randomized controlled trials, and no study investigated cognitive effects following a switch.
The current study is a neuropsychological side study of the Tamoxifen and Exemestane Adjuvant Multinational trial in which the impact of 5 years of adjuvant exemestane (monotherapy) was compared with 2.5-3 years of tamoxifen followed by 2-2.5 years of exemestane (sequential treatment strategy) in postmenopausal hormone receptor-positive early BC patients (12,13). The neuropsychological study included only women who did not receive chemotherapy.
In an earlier publication, short-term (ST) follow-up results of this side study were published (14). We included data of all patients who completed an assessment pre-ET and after 1 year of ET use. We found that at this 1-year follow-up, thus prior to switching, tamoxifen users (n = 80) performed worse on several cognitive domains and reported more attention problems than cancer-free participants (n = 120) and exemestane users (n = 99) (14). Exemestane users did not differ in tested and self-reported cognition from control participants (15).
In the current study, we augment data from the ST follow-up (14) with data from the long-term (LT) follow-up (ie, 5 years of exemestane monotherapy or sequential treatment of tamoxifen followed by exemestane). To make use of the longitudinal character of this study, we will not only use data from the LT follow-up but also use and report on data from the baseline and ST follow-up. In addition, we used data not only from complete cases, as we did in the prior publication (14), but from all cases.
The current study aims, therefore, to describe the cognitive performance of BC patients using all 3 time points (eg, from pre-ET to ST follow-up [1 year after treatment] and LT follow-up [5 years after treatment]). Self-reported outcomes (anxiety and/or depression, menopausal symptoms, fatigue, and cognitive function) are also evaluated. This is the first study that investigates cognitive effects of tamoxifen followed by an AI in BC patients.
Methods
Participants
Participants were Dutch postmenopausal hormone receptor-positive BC patients who participated in the Tamoxifen and Exemestane Adjuvant Multinational trial. They were randomly allocated to either 5 years of adjuvant exemestane (exemestane group) (25 mg/day) or to 2.5-3 years of tamoxifen (20 mg/d) followed by 2-2.5 years of exemestane (25 mg/day; sequential group). Eligibility criteria have been described in detail elsewhere (13). Briefly, patients were included if they had histologically confirmed adenocarcinoma of the breast and positive ER and/or progesterone receptor status and had undergone curative surgery. Additional exclusion criteria for this side study were adjuvant chemotherapy, insufficient command of the Dutch language, central nervous system disease, or signs of dementia according to a dementia screening tool (16). The control group consisted of female friends or relatives without a cancer history of about the same age as the patients. Control participants were included if they had a postmenopausal status, no history of central nervous system disease, sufficient command of the Dutch language, and no signs of dementia according to the dementia screening tool (16). This neuropsychological study was approved by the central review board (Erasmus MC, Rotterdam) and the local medical ethics committees of all participating hospitals. All participants provided written informed consent.
Neuropsychological assessment
We used a battery of 18 cognitive tests that represents 8 cognitive domains (see Table 1) (17-25). All scores were coded such that higher scores indicate better performance.
Table 1.
Summary of cognitive outcome measures
| Cognitive domain | Cognitive tests | Outcome variable | Score range |
|---|---|---|---|
| Verbal memory | Rey auditory verbal learning test (15) Immediate recall | Total of 3 trials | 0-45 |
| Rey auditory verbal learning test (15) Delayed recall | Total for long delay trial | 0-15 | |
| Visual Association Test (16) | Total of 2 trials | 0-24 | |
| Visual memory | Wechsler Memory Scale visual memory subtest (17) Immediate recall | Points awarded according to scoring criteria | 0-41 |
| Wechsler Memory Scale visual memory subtest (17) Delayed recall | Points awarded according to scoring criteria | 0-41 | |
| Information processing speed | Stroop Card 1 (18) | Seconds to complete | ≥0 |
| Stroop Card 2 (18) | Seconds to complete | ≥0 | |
| Trail making test part A (19) | Seconds to complete | ≥0 | |
| Executive functioning | Stroop Card 3 (18) | Seconds to complete | ≥0 |
| Trail making test part B (19) | Seconds to complete | ≥0 | |
| Manual motor speed | Fepsy finger tapping (20) Dominant hand | Mean score of 5 trials of 10 sec | ≥0 |
| Fepsy finger tapping (20) Nondominant hand | Mean score of 5 trials of 10 sec | ≥0 | |
| Verbal fluency | Letter fluency (letters D, A, T) (21) | Total score of 3 letters: 1 min each | ≥0 |
| Category Fluency (animals) (22) | Total score animals: 1 min | ≥0 | |
| Category Fluency (professions) (22) | Total score professions: 1 min | ≥0 | |
| Reaction speed | Fepsy reaction times (20) Dominant hand | Mean milliseconds/30 trials | ≥0 |
| Fepsy reaction times (20) Nondominant hand | Mean milliseconds/30 trials | ≥0 | |
| Working memory | Wechsler Adult Intelligence Scale III Letter-number sequencing (23) | Total correct trials | 0-21 |
Neuropsychological assessments were performed prior to start of ET (baseline), after 1 year (ST follow-up), and at 5 years (LT follow-up).
Patient-reported outcomes
The 25-item Hopkins Symptom Checklist was used to assess anxiety and depression (26). The scale has a 1-week time frame, and the items were rated from “not at all” (1) to “extremely” (4). The outcome variable is the mean of all items (range = 0-4). A mean score was calculated if participants answered 20 or more items (27,28).
The 18-item endocrine subscale of the Functional Assessment of Cancer Therapy-Breast questionnaire was used to assess menopausal symptoms (29). This subscale has a 4-week time frame and consists of 18 items scored on a 5-point scale ranging from “not at all” (0) to “very much” (4). Outcome variable is the sum of reversed scores (0-72) so that higher scores indicate fewer endocrine symptoms. A mean score was calculated if at least half of the items were answered (30).
We used the fatigue symptom scale (3 items) and the cognitive function scale (2 items) of the 30-item European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C30 (EORTC QLQ-C30) version 3.0 (31,32). The scales have a 1-week time frame, and the items were rated from “not at all” (1) to “very much” (4). These scale scores were calculated according to standard EORTC scoring procedures and linearly transformed to a 0-100 scale. Missing values were replaced by the average score of the completed items in the same scale for each individual, provided that at least 50% of the items in that scale had been completed (32). A higher score indicates a higher level of fatigue and a higher level of cognitive functioning.
Statistical analyses
Descriptive statistics were used to characterize the study sample. All raw cognitive test scores were converted into standardized z scores based on the baseline mean and standard deviation of the control group. Eight cognitive domain scores were calculated by the mean of the z scores of the tests that belonged to the particular cognitive domain. The data of all patients and control participants participating in the study were used; attrition patterns across the 3 assessments were compared between groups. We evaluated between-group differences in change over time on anxiety and/or depression, menopausal symptoms, fatigue, and cognitive function. All statistical tests were 2-sided, and statistical significance was set at .05.
To analyze between-group differences in change over time on cognitive test performance, we conducted baseline to follow-up analyses (ST effect: T0 to T1; LT effect: T0 to T2) using a mixed-effects modeling approach with a random intercept, maximum likelihood solution, and autoregressive covariance structure (33). We chose this modeling approach as it can handle missing data, contrary to the earlier publication, in which cases with incomplete observations were discarded because the modeling procedure could not handle missing data. For the primary analyses, the control group was the reference category. If statistically significant, we evaluated differences in mean change from baseline to ST and baseline to LT follow-up between the 2 patient groups and control group and the 2 patient groups.
We investigated the impact of the following possible confounders: age, intelligence quotient (IQ), study attrition, and the time-dependent variables fatigue (EORTC QLQ-C30), menopausal symptoms (Functional Assessment of Cancer Therapy—Breast questionnaire), and anxiety and/or depression (Hopkins Symptom Checklist). We included confounders one by one in the model for every outcome to see if including a confounder would yield a better fit. These models were compared with Bayesian information criterion (BIC) and Akaike information criterion (AIC) (34,35). Models with lower BIC or AIC values are considered better fitting models (36).
Differences in mean change scores over time between the treatment groups and the control group were accompanied by standardized effect sizes (ES) calculated based on the t test statistic: (2*t)/(√degrees of freedom). ES of 0.2 was considered small, 0.5 moderate, and 0.8 large (37).
Analyses were conducted on an intention-to-treat (ITT) basis. Additionally, we performed a per-protocol (PP) analysis on data from patients who met the criteria for minimal adherence with the intervention(s): excluded from the PP analysis were sequential group patients who continued with tamoxifen (instead of switching to exemestane) or switched to exemestane or another AI prematurely; exemestane group patients who switched to tamoxifen; and patients, either from the sequential or exemestane group, who quit prematurely or went without ET more than a month at the time of cognitive assessment.
For all analyses, SPSS for Windows version 27 (IBM Corp, Armonk, NY, USA) was used.
Results
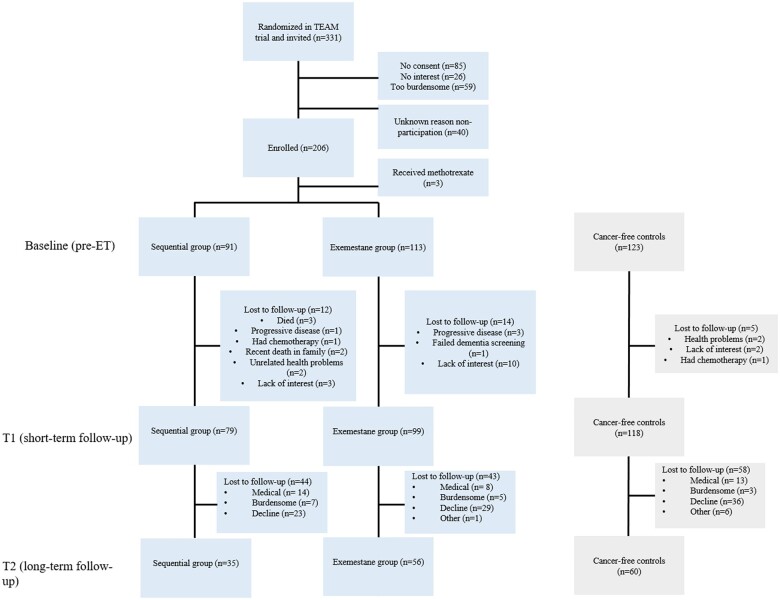
In total, 206 patients (92 patients from the sequential group and 114 patients from the exemestane group) and 124 control women underwent cognitive assessment at baseline. Three (1 sequential group, 1 exemestane group, and 1 control group) of the total 330 participants were undergoing methotrexate (eg, for rheumatism or psoriasis) at baseline and were excluded from analyses. See Figure 1 for the inclusion flowchart.
Figure 1.
Flowchart inclusion neuropsychological side study TEAM trial. ET = endocrine therapy; T1 = 1-year follow-up assessment; T2 = 5-year follow-up assessment; TEAM = Tamoxifen and Exemestane Adjuvant Multinational.
Compared with control participants, the sequential group and the exemestane group were older (P = .01 and P = .02, respectively), and the exemestane group had a lower estimated premorbid IQ (P = .02). See Table 2 for sociodemographic and clinical characteristics of the study population.
Table 2.
Baseline sociodemographic and clinical characteristics of patients receiving tamoxifen followed by exemestane (sequential group), patients receiving only exemestane (exemestane group), and the control group
| Characteristic | Sequential group (n = 91) | Exemestane group (n = 113) | Control group (n = 123) | P |
|---|---|---|---|---|
| Age at random assignment, Mean (SD), y | 69.6 (7.9) | 69.2 (7.0) | 66.9 (8.0) | .02a |
| IQ, Mean (SD) | 100.1 (19.9) | 99.4 (18.8) | 105.3 (19.0) | .04b |
| Time since surgery, Mean (SD), mo | 1.3 (0.7) | 1.5 (0.7) | .13 | |
| Age at menopause, Mean (SD), y | 49.3 (5.3) | 49.7 (4.5) | 48.2 (6.1) | .07 |
| Radiotherapy at baseline or later, No. (%) | 49 (57) | 75 (68) | .17 | |
| Ever use of HRT, No. (%) | 14 (15) | 20 (18) | 23 (19) | .10 |
Post hoc test: sequential group vs control group: P = .01; exemestane group vs control group: P = .02; sequential group vs exemestane group: P = .68. HRT = hormone replacement therapy; IQ = intelligence quotient.
Post hoc test: sequential group vs control group: P = .05; exemestane group vs control group: P = .02; sequential group vs exemestane group: P = .79.
Patient-reported outcomes
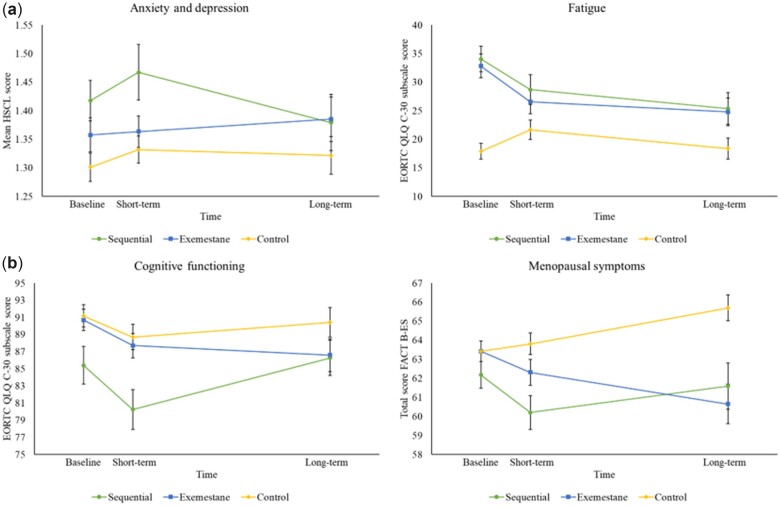
At baseline, the sequential and the exemestane group reported more fatigue compared with the control group (P < .001 for both). This difference diminished over time as fatigue scores decreased (P = .02 and P = .01, respectively). At baseline, the sequential group reported more anxiety and/or depression (P < .001) and endocrine symptoms (P = .03) compared with the control group and more endocrine symptoms compared with the exemestane group (P = .03). During the trial, the exemestane group showed an increase in endocrine symptoms compared with the control group (P = .006). At baseline, the sequential group reported lower cognitive function compared with the control group (P < .001) and the exemestane group (P = .002). Changes over time in self-reported cognitive function did not differ between the sequential group and the exemestane group compared with the control group (P = .79 and P = .45, respectively). The patient-reported outcome scores are depicted in Figure 2.
Figure 2.
Change over time in patient-reported outcomes (anxiety and/or depression, fatigue, cognitive functioning, and menopausal symptoms) in the sequential, the exemestane, and the control group. Anxiety and depression were measured by the HSCL, fatigue, and cognitive functioning by the EORTC QLQ C-30 subscales (fatigue and cognitive functioning) and endocrine symptoms by the FACT B-ES. In Panel a, higher scores represent more complaints. In Panel b, higher scores represent less complaints. EORTC QLQ C-30 = European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C30; FACT B-ES = Functional Assessment of Cancer Therapy-Breast questionnaire; FU = follow-up; HSCL = Hopkins Symptom Checklist.
Compliance to neuropsychological assessment
More women in the sequential and the exemestane group completed only baseline compared with women in the control group (P = .01 and P = .008, respectively). Three dropout patterns were distinguished (see Supplementary Table 1, available online): 1) completed only baseline, 2) completed baseline and first follow-up (T0 and T1), 3) completed all 3 assessments (T0, T1, and T2). Women who completed only baseline and who completed baseline and first follow-up were older than those who completed all assessments (P = .007 and P < .001), and women who completed only baseline had a lower IQ than those who completed all assessments (P = .002), meaning that relatively more younger patients with a higher IQ completed all cognitive assessments. No differences were found between patients from the 3 dropout patterns in anxiety and/or depression, menopausal symptoms, fatigue, and self-reported cognition.
Adherence to trial protocol
See Supplementary Figure 1 (available online) for an overview of participants and dropouts in the PP analyses.
Model selection
For all analyses, adjustment was required based on AIC and BIC values. Most models were at least adjusted for age, IQ, and menopausal symptoms. Between-group differences in general did not change after adjustment. Supplementary Figure 2 (available online) shows the differences per model.
Sequential and exemestane group vs the control group
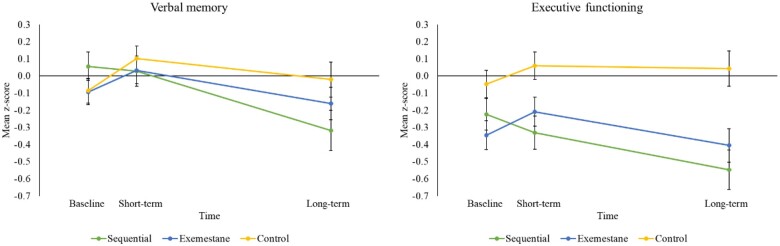
ITT analyses showed that the sequential group had ST and LT decline on verbal memory (ES = 0.26, P = .01; ES = 0.34, P = .003) and executive function (ES = 0.27, P = .007; ES = 0.38, P = .002) compared with controls. The exemestane group did not show decline on any cognitive domain compared with controls. An ST improvement was found on information processing speed for the exemestane group compared with controls (ES = 0.22, P = .02). The ITT results were confirmed in the PP analyses (see Table 3; Supplementary Tables 2 and 3, available online for all results). Figure 3 depicts changes over time for verbal memory and executive function (see Supplementary Figure 3, available online, for changes over time for each cognitive domain).
Table 3.
Results of the intention-to-treat analyses of the sequential, the exemestane, and the control group.
| Adjusted mean z scores |
Sequential group and exemestane vs control |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T0 |
T1 |
T2 |
T0-T1 |
T0-T2 |
|||||||||
| Cognitive domain/test | P overall group by time interaction | Group | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean difference in change over time (95% CI) | P | ESa | Mean difference in change over time (95% CI) | P | ESa | AIC | BIC |
| Verbal memoryb | Sequential | 0.06 (−0.11 to 0.22) | 0.03 (−0.14 to 0.20) | −0.32 (−0.55 to −0.09) | −0.21 (−0.38 to −0.05) | .01 | −0.26 | −0.44 (−0.72 to −0.15) | .003 | −0.34 | |||
| .02 | Exemestane | −0.09 (−0.24 to 0.05) | 0.03 (−0.12 to 0.18) | −0.16 (−0.35 to 0.02) | −0.06 (−0.21 to 0.09) | .42 | −0.08 | −0.13 (−0.39 to 0.12) | .31 | −0.12 | 1375.19 | 1498.98 | |
| Control | −0.09 (−0.23 to 0.06) | 0.10 (−0.04 to 0.24) | −0.02 (−0.22 to 0.18) | ||||||||||
| Visual memoryb | Sequential | −0.38 (−0.58 to −0.19) | −0.36 (−0.57 to −0.15) | −0.37 (−0.63 to −0.11) | 0.03 (−0.18 to 0.23) | .80 | 0.03 | −0.19 (−0.51 to 0.13) | .24 | −0.14 | |||
| .12 | Exemestane | −0.33 (−0.51 to −0.15) | −0.20 (−0.38 to −0.02) | −0.35 (−0.56 to −0.13) | 0.13 (−0.06 to 0.32) | .17 | 0.14 | −0.22 (−0.51 to 0.07) | .14 | −0.18 | 1724.22 | 1848.96 | |
| Control | −0.08 (−0.25 to 0.09) | −0.09 (−0.26 to 0.09) | 0.12 (−0.11 to 0.35) | ||||||||||
| Information processing speedb | Sequential | −0.24 (−0.39 to −0.09) | −0.28 (−0.44 to −0.12) | −0.44 (−0.64 to −0.25) | −0.05 (−0.19 to 0.09) | .50 | −0.07 | −0.05 (−0.29 to 0.18) | .67 | −0.05 | |||
| .04 | Exemestane | −0.23 (−0.36 to −0.09) | −0.06 (−0.20 to 0.08) | −0.35 (−0.51 to −0.19) | 0.15 (0.02 to 0.28) | .02 | 0.22 | 0.02 (−0.19 to 0.24) | .82 | 0.03 | 1261.08 | 1385.96 | |
| Control | −0.03 (−0.16 to 0.10) | −0.02 (−0.15 to 0.11) | −0.18 (−0.35 to −0.01) | ||||||||||
| Executive functioningc | Sequential | −0.22 (−0.41 to −0.04) | −0.33 (−0.52 to −0.14) | −0.55 (−0.77 to −0.32) | −0.22 (−0.37 to −0.06) | .007 | −0.27 | −0.41 (−0.68 to −0.15) | .002 | −0.38 | |||
| .005 | Exemestane | −0.35 (−0.51 to −0.18) | −0.21 (−0.38 to −0.04) | −0.41 (−0.60 to −0.21) | 0.03 (−0.11 to 0.17) | .69 | 0.04 | −0.15 (−0.39 to 0.09) | .22 | −0.15 | 1475.47 | 1623.23 | |
| Control | −0.05 (−0.20 to 0.11) | 0.06 (−0.10 to 0.22) | 0.04 (−0.16 to 0.24) | ||||||||||
| Motor speedd | Sequential | −0.02 (−0.18 to 0.14) | −0.01 (−0.17 to 0.16) | −0.22 (−0.41 to −0.02) | −0.08 (−0.22 to 0.06) | .25 | −0.11 | −0.21 (−0.43 to 0.02) | .07 | −0.22 | |||
| .31 | Exemestane | −0.05 (−0.20 to 0.09) | 0.01 (−0.14 to 0.16) | −0.22 (−0.39 to −0.06) | −0.03 (−0.16 to 0.10) | .62 | −0.05 | −0.18 (−0.39 to 0.02) | .08 | −0.22 | 1292.93 | 1417.82 | |
| Control | −0.08 (−0.21 to 0.06) | 0.02 (−0.12 to 0.16) | −0.07 (−0.23 to 0.10) | ||||||||||
| Verbal fluencye | Sequential | −0.39 (−0.53 to −0.25) | −0.39 (−0.54 to −0.25) | −0.39 (−0.57 to −0.21) | −0.02 (−0.15 to 0.10) | .70 | −0.04 | 0.06 (−0.12 to 0.25) | .49 | 0.09 | |||
| .76 | Exemestane | −0.42 (−0.54 to −0.29) | −0.37 (−0.50 to −0.24) | −0.48 (−0.63 to −0.34) | 0.03 (−0.09 to 0.14) | .66 | 0.04 | 0.00 (−0.16 to 0.16) | >.99 | 0.00 | 1140.02 | 1242.04 | |
| Control | −0.09 (−0.21 to 0.03) | −0.07 (−0.19 to 0.05) | −0.16 (−0.30 to −0.01) | ||||||||||
| Reaction speedf | Sequential | −0.27 (−0.47 to −0.06) | −0.40 (−0.62 to −0.18) | −0.27 (−0.55 to 0.00) | −0.12 (−0.35 to 0.11) | .305 | −0.10 | 0.05 (−0.30 to 0.40) | .76 | 0.04 | |||
| .523 | Exemestane | −0.22 (−0.40 to −0.03) | −0.16 (−0.36 to 0.03) | −0.25 (−0.49 to −0.02) | 0.06 (−0.15 to 0.27) | .573 | 0.06 | 0.02 (−0.30 to 0.35) | .89 | 0.02 | 1813.94 | 1938.06 | |
| Control | 0.02 (−0.16 to 0.19) | 0.01 (−0.17 to 0.19) | −0.05 (−0.30 to 0.20) | ||||||||||
| Working memorye | Sequential | −0.33 (−0.51 to −0.15) | −0.16 (−0.35 to 0.03) | −0.43 (−0.70 to −0.15) | 0.12 (−0.14 to 0.38) | .36 | 0.09 | −0.23 (−0.62 to 0.16) | .24 | −0.13 | |||
| .21 | Exemestane | −0.30 (−0.46 to −0.13) | −0.20 (−0.37 to −0.03) | −0.45 (−0.67 to −0.24) | 0.04 (−0.20 to 0.29) | .72 | 0.03 | −0.30 (−0.65 to 0.05) | .10 | −0.19 | 1833.39 | 1935.06 | |
| Control | −0.06 (−0.21 to 0.10) | −0.01 (−0.16 to 0.15) | 0.08 (−0.14 to 0.31) | ||||||||||
Effect sizes: 0.20 small effect, 0.50 moderate effect, 0.80 large effect. AIC = Akaike information criterion; BIC = Bayesian information criterion; CI = confidence interval; ES = effect size; FACT-ES = Functional Assessment of Cancer Therapy-Breast questionnaire; HSCL = Hopkins Symptom Checklist; IQ = intelligence quotient; T0 = baseline; T1 = 1-year follow-up; T2 = 5-year follow-up.
Adjusted for age, IQ, FACT-ES.
Adjusted for age, IQ, HSCL, FACT-ES.
Adjusted for age, IQ, HSCL.
Adjusted for age, IQ.
Adjusted for IQ, HSCL, FACT-ES.
Figure 3.
Adjusted standardized change over time on verbal memory and executive function between patients and control participants. Mean z scores are depicted per cognitive domain.
Sequential group vs exemestane group
The sequential group showed an ST decline on information processing speed (ES = 0.33, P = .01) and executive function (ES = 0.32, P = .01) and an LT decline on verbal memory (ES = 0.33, P = .02) compared with the exemestane group. The ITT results were confirmed in the PP analyses (see Supplementary Tables 4 and 5, available online).
Discussion
Our earlier findings suggested cognitive adverse effects of tamoxifen and no effects of exemestane in a 1-year follow-up study in postmenopausal early hormone receptor-positive BC patients (14). The current study augments these data by evaluating ST and LT cognitive effects of tamoxifen followed by exemestane and exemestane using all data and time points. After controlling for age, IQ, attrition patterns, menopausal symptoms, anxiety and/or depression, and/or fatigue, tamoxifen and tamoxifen followed by exemestane were associated with decline in verbal memory and executive functioning. Observed effects were of small magnitude. Treatment with exemestane only was not associated with cognitive decline. The ITT and PP analyses yielded comparable results. We found no differences between the patient groups and the control group in changes over time in self-reported cognitive function and anxiety and/or depression. Differences in self-reported fatigue at baseline diminished over time in both patient groups compared with the control group. The exemestane group reported more menopausal symptoms over time than the control group.
The observation of tamoxifen’s small cognitive effects on verbal memory and executive functioning is in line with an emerging body of (predominantly cross-sectional) studies reporting adverse effects of tamoxifen (38). Our observation of (small) adverse effects of tamoxifen alone and tamoxifen followed by exemestane, combined with the absence of any effect of exemestane monotherapy, suggests a potential carryover effect of tamoxifen. Cognitive effects of tamoxifen followed by another agent have not been examined previously. A small imaging study showed that tamoxifen was associated with structural brain changes [eg, smaller hippocampal volumes (39)], which could partly explain LT effects of tamoxifen.
The impact of ET on cognition and brain health is poorly studied and incompletely understood, both from a preclinical and clinical perspective (10,11). The mixed findings in the literature do not give clear direction for interpreting our results. Several important differences between tamoxifen and exemestane may have contributed to our observations, which could also provide guidance to future research initiatives. First, AIs inactivate aromatase, thereby preventing conversion of androgens into estrogens. Tamoxifen, however, competitively binds to ERs. Second, tamoxifen has anti-estrogenic effects on breast tissue but does not act as an anti-estrogen in all tissues (40-42). Whether tamoxifen has an estrogenic or anti-estrogenic effect (or both) on the brain is unknown. The absence of cognitive effects of exemestane in our study may suggest that further downregulation of estrogen production in already postmenopausal women does not impact cognition. Also, ERα can be activated ligand independently, without estrogens by growth factors such as insulin-like growth factor 1 (43). ERs can still exert some transcriptional actions during exemestane use, in contrast to tamoxifen use. This might have contributed to the absence of cognitive changes following exemestane compared with tamoxifen. In addition, exemestane and its metabolites have a mild androgenic property that could be protective for cognition (44). Our findings warrant further fundamental research to characterize the influence of tamoxifen as estrogenic, anti-estrogenic, or maybe of a different character.
To better understand the cause of cognitive effects of tamoxifen, it could be useful to study the pharmacokinetics of tamoxifen in relation to cognition. Tamoxifen is a prodrug that exerts its effects only after conversion to active metabolites mainly by the liver (45). Therefore, focusing on the relation between tamoxifen’s metabolites (eg, endoxifen) and cognition can lead to a more direct examination of causality. Our research group is initiating a substudy of the Therapeutic drug monitoring Of TAMoxifen (TOTAM) trial (Netherlands Trial Register NL6919/NTR7113) on dose- and serum-dependent cognitive effects of tamoxifen and its metabolites.
A limitation of this study is the small sample size at the LT follow-up, reducing the statistical power of the LT evaluation. Results of the LT evaluation should be viewed as hypothesis generating and need to be confirmed in larger studies. The third cognitive assessment was not part of the original study protocol, which may have contributed to a lower accrual rate. Another limitation is that in both patient groups, vulnerable patients (of older age and with lower IQ) dropped out early, which could have biased the findings. The strengths of the current study include the prospective nature, the inclusion of chemotherapy-naïve patients only, and a control group of women without a cancer history.
In conclusion, our results confirm our previous ST findings and add to these by showing that sequential treatment with tamoxifen and exemestane was associated with ST and LT decline on several tested cognitive functions, whereas exemestane only was not. The modest adverse effects of tamoxifen and tamoxifen followed by exemestane occurred in absence of treatment-specific changes in self-reported cognitive symptoms. As cognitive test performance is associated with outcomes such as financial management, employability, and medication management, adverse effects, even modest effects, could be of clinical relevance (46). Studies with additional measures are needed to investigate the impact on real-world performance. Given the large group of women receiving ET, the results underline the clinical need for well-controlled, prospective trials.
Supplementary Material
Acknowledgements
The funders did not play a role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Contributor Information
Philippe R Lee Meeuw Kjoe, Department of Psychosocial Research and Epidemiology, The Netherlands Cancer Institute, Amsterdam, The Netherlands.
Jacobien M Kieffer, Department of Psychosocial Research and Epidemiology, The Netherlands Cancer Institute, Amsterdam, The Netherlands.
Brent J Small, School of Aging Studies, University of South Florida, Tampa, FL, USA.
Willem Boogerd, Department of Neuro-oncology, The Netherlands Cancer Institute, Amsterdam, The Netherlands.
Christina M Schilder, Department of Psychosocial Research and Epidemiology, The Netherlands Cancer Institute, Amsterdam, The Netherlands.
Elsken van der Wall, Department of Medical Oncology, University Medical Center Utrecht, Utrecht, The Netherlands.
Elma Meershoek-Klein Kranenbarg, Department of Surgery, Leiden University Medical Center, Leiden, The Netherlands.
Cornelis J H van de Velde, Department of Surgery, Leiden University Medical Center, Leiden, The Netherlands.
Sanne B Schagen, Department of Psychosocial Research and Epidemiology, The Netherlands Cancer Institute, Amsterdam, The Netherlands; Department of Psychology, University of Amsterdam, Amsterdam, The Netherlands.
Data availability
Data supporting the findings of this study are available in the NKI repository. The data can also be shared upon request to the corresponding author.
Author contributions
Philippe R. Lee Meeuw Kjoe, MSc (Data curation; Formal analysis; Validation; Writing - original draft; Writing - review & editing); Jacobien M. Kieffer, PhD (Data curation; Formal analysis; Validation; Writing - original draft); Brent J. Small, PhD (Writing - original draft); Willem Boogerd, MD PhD (Conceptualization; Methodology; Writing - original draft); Christina M. Schilder, PhD (Conceptualization; Data curation; Investigation; Methodology; Project administration; Writing - original draft); Elsken van der Wall, MD PhD (Writing - original draft; Writing - review & editing); Elma Meershoek-Klein Kranenbarg, MSc (Conceptualization; Methodology; Writing - original draft); Cornelis J.H. van de Velde, MD PhD (Conceptualization; Methodology; Writing - original draft); and Sanne B. Schagen, PhD (Conceptualization; Funding acquisition; Methodology; Resources; Supervision; Writing - original draft; Writing - review & editing).
Funding
This work was supported by the Dutch Cancer Society, KWF Kankerbestrijding [grant number KWF 2015-7937]. The baseline and short-term follow-up measurement were supported by an independent research grant from Pfizer.
References
- 1. Early Breast Cancer Trialists’ Collaborative Group. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386(10001):1341-1352. [DOI] [PubMed] [Google Scholar]
- 2. Norbury R, Cutter WJ, Compton J, et al. The neuroprotective effects of estrogen on the aging brain. Exp Gerontol. 2003;38(1-2):109-117. [DOI] [PubMed] [Google Scholar]
- 3. Turgeon JL, Carr MC, Maki PM, et al. Complex actions of sex steroids in adipose tissue, the cardiovascular system, and brain: insights from basic science and clinical studies. Endocr Rev. 2006;27(6):575-605. [DOI] [PubMed] [Google Scholar]
- 4. Maki PM, Dumas J.. Mechanisms of action of estrogen in the brain: insights from human neuroimaging and psychopharmacologic studies. Semin Reprod Med 2009;27(3):250-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Van Dyk K, Crespi CM, Bower JE, et al. The cognitive effects of endocrine therapy in survivors of breast cancer: a prospective longitudinal study up to 6 years after treatment. Cancer. 2019;125(5):681-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jenkins V, Shilling V, Fallowfield L, et al. Does hormone therapy for the treatment of breast cancer have a detrimental effect on memory and cognition? A pilot study. Psychooncology. 2004;13(1):61-66. [DOI] [PubMed] [Google Scholar]
- 7. Phillips K-A, Ribi K, Sun Z, et al. Cognitive function in postmenopausal women receiving adjuvant letrozole or tamoxifen for breast cancer in the BIG 1-98 randomized trial. Breast. 2010;19(5):388-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Le Rhun E, Delbeuck X, Lefeuvre-Plesse C, et al. A phase III randomized multicenter trial evaluating cognition in post-menopausal breast cancer patients receiving adjuvant hormonotherapy. Breast Cancer Res Treat. 2015;152(3):569-580. [DOI] [PubMed] [Google Scholar]
- 9. Jenkins VA, Ambroisine LM, Atkins L, et al. Effects of anastrozole on cognitive performance in postmenopausal women: a randomised, double-blind chemoprevention trial (IBIS II). Lancet Oncol. 2008;9(10):953-961. [DOI] [PubMed] [Google Scholar]
- 10. Haggstrom LR, Vardy JL, Carson EK, et al. Effects of endocrine therapy on cognitive function in patients with breast cancer: a comprehensive review. Cancers. 2022;14(4):920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee Meeuw Kjoe PR, van der Wall E, Schagen SB.. Endocrine therapy with or without CDK4/6 inhibitors in women with hormone-receptor positive breast cancer: what do we know about the effects on cognition? Clin Breast Cancer. 2022;22(3):191-199. [DOI] [PubMed] [Google Scholar]
- 12. van de Velde CJH, Rea D, Seynaeve C, et al. Adjuvant tamoxifen and exemestane in early breast cancer (TEAM): a randomised phase 3 trial. Lancet. 2011;377(9762):321-331. [DOI] [PubMed] [Google Scholar]
- 13. Schilder CM, Eggens PC, Seynaeve C, et al. Neuropsychological functioning in postmenopausal breast cancer patients treated with tamoxifen or exemestane after AC-chemotherapy: cross-sectional findings from the neuropsychological TEAM- side study. Acta Oncol. 2009;48(1):76-85. [DOI] [PubMed] [Google Scholar]
- 14. Schilder CM, Seynaeve C, Beex LV, et al. Effects of tamoxifen and exemestane on cognitive functioning of postmenopausal patients with breast cancer: results from the neuropsychological side study of the tamoxifen and exemestane adjuvant multinational trial. J Clin Oncol. 2010;28(8):1294-1300. [DOI] [PubMed] [Google Scholar]
- 15. Schilder CM, Seynaeve C, Linn SC, et al. Self‐reported cognitive functioning in postmenopausal breast cancer patients before and during endocrine treatment: findings from the neuropsychological TEAM side-study. Psychooncology. 2012;21(5):479-487. [DOI] [PubMed] [Google Scholar]
- 16. Meulen EFJ, Schmand B, van Campen JP, et al. The seven minute screen: a neurocognitive screening test highly sensitive to various types of dementia. J Neurol Neurosurg Psychiatry. 2004;75(5):700-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Van den Burg W, Saan RJ, Deelman BG.. 15-Woordentest: Provisional Manual. Groningen: University Hospital, Department of Neuropsychology;1985. [Google Scholar]
- 18. Lindeboom J, Schmand B, Tulner L, et al. Visual association test to detect early dementia of the Alzheimer type. J Neurol Neurosurg Psychiatry. 2002;73(2):126-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wechsler D. Wechsler Memory Scale-Revised. San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- 20. Hammes JG. De Stroop Kleur-Woord Test. Amsterdam: Harcourt Test Publ; 1978. [Google Scholar]
- 21. Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8(3):271-276. [Google Scholar]
- 22. Alpherts W, Aldenkamp AP.. FePsy: The Iron Psyche. Heemstede: Instituut voor epilepsiebestrijding; 1994. [Google Scholar]
- 23. Van Der Elst WI, Van Boxtel MP, Van Breukelen GJ, et al. Normative data for the Animal, Profession and Letter M Naming verbal fluency tests for Dutch speaking participants and the effects of age, education, and sex. J Int Neuropsychol Soc. 2006;12(1):80-89. [DOI] [PubMed] [Google Scholar]
- 24. Lezak MD, Howieson DB, Loring DW, et al. Neuropsychological Assessment. New York: Oxford University Press; 2004. [Google Scholar]
- 25. Wechsler D. WAIS-III Nederlandstalige Bewerking. Technische Handleiding. Lisse, The Netherlands: Swets & Zeitlinger; 2000. [Google Scholar]
- 26. Fröjdh K, Håkansson A, Karlsson I.. The Hopkins Symptom Checklist-25 is a sensitive case-finder of clinically important depressive states in elderly people in primary care. Int J Geriat Psychiatry. 2004;19(4):386-390. [DOI] [PubMed] [Google Scholar]
- 27. Nettelbladt P, Hansson L, Stefansson CG, et al. Test characteristics of the Hopkins Symptom Check List-25 (HSCL-25) in Sweden, using the Present State Examination (PSE-9) as a caseness criterion. Soc Psychiatry Psychiatr Epidemiol. 1993;28(3):130-133. [DOI] [PubMed] [Google Scholar]
- 28. Sandanger I, Moum T, Ingebrigtsen G, et al. The meaning and significance of caseness: the Hopkins Symptom Checklist-25 and the Composite International Diagnostic Interview II. Soc Psychiatry Psychiatr Epidemiol. 1999;34(1):53-59. [DOI] [PubMed] [Google Scholar]
- 29. Fallowfield LJ, Leaity SK, Howell A, Benson S, Cella D.. Assessment of quality of life in women undergoing hormonal therapy for breast cancer: validation of an endocrine symptom subscale for the FACT-B. Breast Cancer Res Treat. 1999;55(2):187-197. [DOI] [PubMed] [Google Scholar]
- 30. Cella D. FACIT Manual: Manual of the Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System. Evanston, IL: CORE; 1997. [Google Scholar]
- 31. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365-376. [DOI] [PubMed] [Google Scholar]
- 32. Fayers PM, Aaronson NK, Bjordal K, et al. EORTC QLQ-C30 Scoring Manual. 3rd ed. Brussels: European Organization for Research and Treatment of Cancer; 2001. [Google Scholar]
- 33. Littell RC, Pendergast J, Natarajan R.. Modelling covariance structure in the analysis of repeated measures data. Statist Med. 2000;19(13):1793-1819. [DOI] [PubMed] [Google Scholar]
- 34. Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6(2):461-464. [Google Scholar]
- 35. Akaike H. Information theory and an extension of the maximum likelihood principle. In: Petrov BN, Caski F, eds. Proceedings of the Second International Symposium on Information Theory. Budapest: Akademiai Kiado; 1973:267-281. [Google Scholar]
- 36. Raftery A. Bayesian model selection in social research. Sociol Methodol. 1995;25:111-163. [Google Scholar]
- 37. Cohen J. Statistical Power Analysis for the Behavioural Sciences. Revised ed.New York: Academic Press; 1977. [Google Scholar]
- 38. Jebahi F, Sharma S, Bloss JE, et al. Effects of tamoxifen on cognition and language in women with breast cancer: a systematic search and a scoping review. Psycho‐Oncology. 2021;30(8):1262-1277. [DOI] [PubMed] [Google Scholar]
- 39. Eberling JL, Wu C, Tong-Turnbeaugh R, et al. Estrogen- and tamoxifen-associated effects on brain structure and function. Neuroimage. 2004;21(1):364-371. [DOI] [PubMed] [Google Scholar]
- 40. Newhouse P, Albert K, Astur R, et al. Tamoxifen improves cholinergically modulated cognitive performance in postmenopausal women. Neuropsychopharmacol. 2013;38(13):2632-2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang Z, Park JW, Ahn IS, et al. Estrogen receptor alpha in the brain mediates tamoxifen-induced changes in physiology in mice. Elife. 2021;10:e63333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lichtenfels M, da Silva Dornelles A, dos Santos Petry F, et al. The anticancer estrogen receptor antagonist tamoxifen impairs consolidation of inhibitory avoidance memory through estrogen receptor alpha. J Neural Transm. 2017;124(11):1331-1339. [DOI] [PubMed] [Google Scholar]
- 43. Baumgartner NE, Daniel JM.. Estrogen receptor α: a critical role in successful female cognitive aging. Climacteric. 2021;24(4):333-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hirshman E, Merritt P, Wang CC, et al. Evidence that androgenic and estrogenic metabolites contribute to the effects of dehydroepiandrosterone on cognition in postmenopausal women. Horm Behav. 2004;45(2):144-155. [DOI] [PubMed] [Google Scholar]
- 45. Jordan VC. Tamoxifen: a most unlikely pioneering medicine. Nat Rev Drug Discov. 2003;2(3):205-213. [DOI] [PubMed] [Google Scholar]
- 46. Zwart W, Terra H, Linn SC, Schagen SB.. Cognitive effects of endocrine therapy for breast cancer: keep calm and carry on? Nat Rev Clin Oncol. 2015;12(10):597-606. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting the findings of this study are available in the NKI repository. The data can also be shared upon request to the corresponding author.