Abstract
Glycocalyx is located outside the vascular endothelial cells playing an important role in vascular homeostasis. However, lacking efficient detection methods is one of the biggest obstacles to study the glycocalyx. In this study, three dehydration methods were used to compare the preservation of HUVEC, aorta and kidney glycocalyx by transmission electron microscope. The chemical pre-fixation was performed by lanthanum nitrate staining, and the mice aorta and renal glycocalyx were prepared by different dehydration methods such as ethanol gradient, acetone gradient and low temperature dehydration. HUVEC glycocalyx was prepared by acetone gradient and low temperature dehydration. Low temperature dehydration method preserves HUVEC and mice aortic glycocalyx completely, which had a certain thickness and presented a needle-like structure. But for mice kidney, the acetone gradient dehydration preparation method could better preserve the glycocalyx integrity than other two methods. In conclusion, low temperature dehydration method is suitable for HUVEC and aortic glycocalyx preservation, acetone gradient dehydration method is more suitable for kidney glycocalyx preservation.
Keywords: Glycocalyx, Dehydration, Transmission electron microscopy
1. Introduction
The endothelial glycocalyx is located outside the vascular endothelial cells between the blood and blood vessels [[1], [2], [3]]. Glycocalyx is a layer of villi-like polysaccharide protein complex structure, which is a dynamic natural barrier on the surface of endothelial cells [[3], [4], [5]]. Glycocalyx is a carbohydrate-rich layer present on the luminal surface of all endothelial cells and consists mainly of glycoproteins and proteoglycans [[5], [6], [7]]. Proteoglycans are composed of core proteins (such as syndecan) and glycosaminoglycans (such as heparan sulfate, chondroitin sulfate, dermatan sulfate, keratan sulfate, and hyaluronic acid) [6,[8], [9], [10], [11], [12]]. The endothelial glycocalyx is mostly found on endothelial cells between the blood and the intima surface of the blood vessel. The endothelial glycocalyx plays an important role in maintaining vascular permeability, responding to mechanotransduction and conducting signal transduction [6,13]. In addition, glycocalyx also exists on the surface of kidney podocytes. The kidney glycocalyx structure is the basis for maintaining the structure of foot process and gap membrane [[14], [15], [16], [17]]. Therefore, the glycocalyx has important physiological and pathological significance, and better preservation glycocalyx complete structure is benefit for the study of various diseases. Vascular complications closely associate with glycocalyx degradation [6,13,18]. Glycocalyx injury leads to the increase of vascular permeability [19,20], which accelerate the formation of capillary leakage and edema [21], and lead to vascular endothelial dysfunction [22]. What's more, the glycocalyx damage affects the leukocytes adhesion [23], thus increasing the sensitivity of vascular system to atherogenic stimulation [24,25]. Therefore, observation of intact glycocalyx is necessary for disease prediction. And optimization of sample preparation technology is very important for preservation of glycocalyx completely in vitro. There are many methods for dyeing and preservation of glycocalyx. One staining method is used for the staining both endothelial glycocalyx of large vessels and the staining of endothelial glycocalyx of microvessels, but the staining effect is not well satisfactory. So until now, it is not clear which method is more suitable for the staining of the endothelial glycocalyx of large vessels or microvessels. For example, Luft et al. [26] observed glycocalyx structures on rat mesenteric capillaries using Liao red staining and transmission electron microscopy. Dogne et al. [27] observed mice myocardial arteriole glycocalyx using alcian blue 8 GX staining and transmission electron microscopy. Ramnath and Oltean used Alcian blue to label the kidney glycocalyx [28]. Liu Zengbo et al. [29] observed mice glomerular glycocalyx by lanthanum nitrate staining and transmission electron microscopy. Barua D et al. used lanthanum to label glycocalyx of Xenopus gastrula tissues [30].
However, all these methods mentioned are based on the glycocalyx structural integrity. Because the glycocalyx structure is easily damaged and lost during fixation and staining procedures, the experimenter cannot observe the complete structure of the glycocalyx well. That has deeply troubled the research work of relevant scientific workers. Using intravital microscopy, Vink et al. [31] observed the endothelial glycocalyx of hamster cremaster muscle capillaries in vivo by an indirect method. However, intravital microscopy-based methods for estimating glycocalyx thickness are indirect. And intravital microscopy cannot be used to image the endothelial glycocalyx in larger vessels. With high resolution, electron microscopy can directly observe various glycocalyx forms. Therefore, it's important to find proper ways to preserve the glycocalyx structure in staining process. Previous report pointed out that ethanol gradient dehydration can lead to glycocalyx water content decrease resulting in glycocalyx observed range reduction [32]. They propose that the intact structure of the glycocalyx can be preserved by rapid freezing/freeze substitution transmission electron microscopy. In the process of low temperature dehydration, ice crystals wouldn't form to destroy the glycocalyx structure, and water content wouldn't decrease in the tissue. The BAEC glycocalyx preserved by the traditional dehydration method with alcohol as a substitute was only 0.04 μm thick. But the BAEC glycocalyx dehydrated by acetone, using rapid freezing/freeze substitution, was more intact and the thickness was up to 11 μm [32]. Vonschack ML et al. believed that acetone was used as a substituent and the fixed method of freeze substitution could preserve fine cellular ultrastructure [33]. This is because glycocalyx was preserved in vitrified water (noncrystalline glassyice),which was permited the glycocalyx and other hydrophilic cellular structures to remain configured as if alive [34]. Therefore, in this paper, HUVEC and mice aorta/kidney glycocalyx were chemically pre-fixed by lanthanum nitrate staining method, and then they were dehydrated by ethanol gradient, acetone gradient or low temperature dehydration method to observe the glycocalyx using transmission electron microscopy. The aim of this study is to explore the effects of different dehydration methods on the structural integrity of HUVEC/mice endothelial glycocalyx, so as to screen the best way to preserve glycocalyx in vitro.
2. Materials and methods
2.1. Main reagents and their preparation
Lanthanum nitrate (GR, Macklin, China); sodium cacodylate (GR, Macklin, China); 50% glutaraldehyde (GR, Macklin, China); paraformaldehyde (GR, sinopharm, China).
2% lanthanum nitrate perfusion solution (100 mL): 2 g of lanthanum nitrate + 100 mL of 0.2 mol/L sodium cacodylate-HCl buffer, adjust the pH to 7.1.
2% lanthanum nitrate fixative solution (100 mL): 2 g of lanthanum nitrate + 5 mL of 50% glutaraldehyde solution + 2 g of paraformaldehyde + 100 mL of 0.2 mol/L sodium cacodylate-HCl buffer, adjust the pH value to 7.1.
0.2 mol/L sodium cacodylate-HCl buffer (100 mL): 0.2 mol/L sodium cacodylate 25 mL + 0.2 mol/L HCl 2.1 mL + deionized water 72.9 mL.
2% lanthanum nitrate rinse solution (100 mL): 2 g of lanthanum nitrate + 100 mL of 0.2 mol/L sodium cacodylate-HCl buffer, adjust the pH to 7.1.
2.2. Animal and groups
Four male C57BL/6 J mice at 8 weeks of age, were purchased from SPF (Beijing) Biotechnology Co., LTD (Beijing, China). The animals were housed in an air-conditioned room at 23 ± 2 °C with a 12-h dark/light cycle. All animals unlimited access to food and water. All animals received human care in accordance with the recommendations of the National Institutes of Health Guide for Care and Use of Laboratory Animals, and the experiment scheme was approved by the Committee on the Ethics of Animal Experiments of Institute of Oceanology, Chinese Academy of Sciences. The IACUC approval number is CTEC-2021 (02–01). Four mice were used to compare the effects of different dehydration methods on glycocalyx preparation in vitro. Both the aortas and corticomedulary junction in kidney of the same healthy mice were divided into three equal parts. One part was dehydrated by gradient with ethanol, the second part was dehydrated by gradient with acetone, and the third part was dehydrated by freezing substitution method, other processing steps were the same.
2.3. Sample preparation method
2.3.1. Chemical fixation
The mice were anesthetized by intraperitoneal anesthesia, the right ventricle was bled, the left ventricle was perfused with 2% lanthanum nitrate perfusion solution at a flow rate of 1 mL/min until the kidneys became white, and then perfused with 2% lanthanum nitrate fixative solution. Then the mice aortic and kidney were collected. The mice aorta or kidney tissue, about 1 mm3 in size, was placed in lanthanum nitrate fixative. HUVEC was rinsed three times with PBS and fixed in 2% lanthanum nitrate fixative for 2 h.
Then the specimens were rinsed 4 times, 15 min each time, with 2% lanthanum nitrate rinsing solution. Then the specimens were fixed with 1% osmic acid fixative solution for 2 h; and then the specimens were rinsed 4 times with 2% lanthanum nitrate rinsing solution for 15 min each time.
2.3.2. Three different dehydration methods
2.3.2.1. Ethanol gradient dehydration
After fixation, the specimens were dehydrated in gradients of 30%, 50%, 70%, 80%, 90% and 100% ethanol, each gradient was dehydrated for 15 min.
2.3.2.2. Acetone gradient dehydration
After fixation, the specimens were dehydrated by 30%, 50%, 70%, 80%, 90% and 100% acetone, each gradient was dehydrated for 15 min.
2.3.2.3. Low temperature dehydration
After fixation, the specimens were dehydrated in freeze substitute(Leica EM AFS2)by 30% acetone for 15 min at 0 °C; Then the specimens were dehydrated by 50% acetone for 30 min, temperature drops from 0 °C to −20 °C; At last, the specimens were dehydrated by 70%, 85%, 95%, and 100% acetone for 30 min each at −20 °C.
2.3.3. Embedding
The specimens that using ethanol gradient dehydration were removed ethanol by using 100% ethanol:100% acetone (1:1) and twice 100% acetone for 15 min in each step. After dehydration, the specimens were soaked in 100% acetone: resin (Spon812, SPI, United States) = 2:1 at room temperature for 6 h; Then the specimens were soaked in 100% acetone: resin = 1:1 at room temperature for 6 h; Then the specimens were soaked in 100% acetone: resin = 1:2 at room temperature for 6 h; At last, the specimens were soaked twice in pure resin for 1.5 h each time at room temperature. Then, the specimens were placed in an embedded mold, transferred to an oven at 37 °C, 45 °C and 60 °C for 24 h in each temperature gradient.
2.3.4. Ultrathin sectioning and staining
The ultrathin microtome (Leica, UC7, Germany) was semi-thinly positioned to the vascular area, and the ultrathin section was 70 nm; The samples were then stained with 2% uranyl acetate-lead citrate double staining; transmission electron microscopy (Hitachi, HT7700, Japan) was used for samples observation and image acquisition.
2.4. Cell culture and sample preparation
Human Umbilical Vein Endothelial Cells (HUVECs) were purchased from American Type Culture Collection. Cells were cultured in DMEM (Hyclone, Cytiva, China) with 10% fetal bovine serum (ExCell Bio, Jiangsu, China) at 37 °C with 5% CO2.
HUVEC glycocalyx samples were prepared by acetone gradient dehydration and low temperature dehydration. For details, see 1.3.
2.5. Immunofluorescence
WGA (Wheat Germ Agglutinin) specifically binds N-acetylglucosamine and N-acetylneuraminic acid, and has been used in many studies to label glycocalyx [[35], [36], [37]]. The mouse kidney/aorta were incubated in WGA (Wheat Germ Agglutinin-iFluor 488, AAT Bioquest) and DAPI(Beijing Solarbio Science & Technology Co., Ltd.). Images were taken on a Leica Mica confocalmicroscope (Biomark Biology, China).
HUVEC cells were cultured in confocal dishes (NEST Biotechnology, Jiangsu, China), blocked by BSA (Yeasen, Shanghai, China.), incubated in WGA (Wheat Germ Agglutinin-iFluor 488, AAT Bioquest), DIL (Beijing Solarbio Science & Technology Co., Ltd.) and DAPI(Beijing Solarbio Science & Technology Co., Ltd.). Images were taken on a Carl Zeiss LSM 710 confocal microscope (Carl Zeiss, Jena, Germany).
2.6. Glycocalyx depth calculation
Image J was used to calculate that the depth of the “substance” outside endothelial cells which was the glycocalyx depth. These are shown by orange lines in Fig. 1, Fig. 2A.
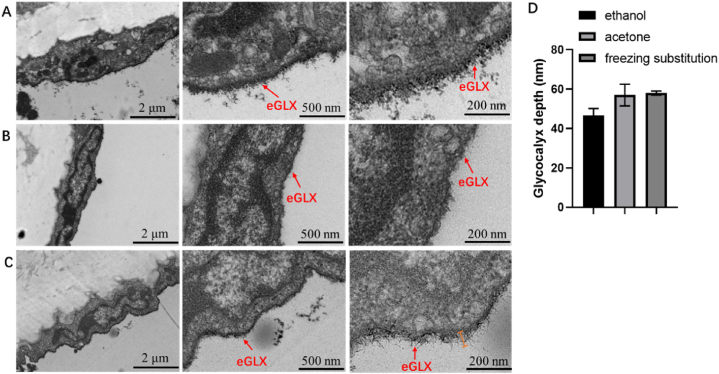
Fig. 1.
Transmission electron microscopy images of the aortic glycocalyx in the same mice. A: Gradient dehydration with ethanol; B: Gradient dehydration with acetone; C: Using low temperature dehydration method; D: Glycocalyx depth covering the aorta endothelial cell surface quantified in electron microscopic images from healthy mice in three different dehydration methods. eGLX: endothelial glycocalyx.
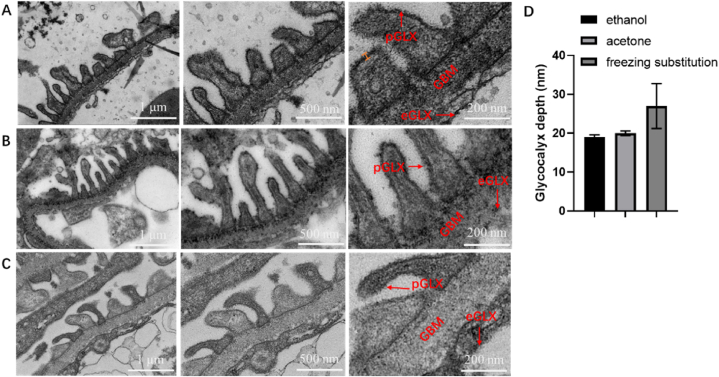
Fig. 2.
Transmission electron microscope images of mice glomerular glycocalyx. A: Using ethanol gradient dehydration method; B: Using acetone gradient dehydration method; C: Using low temperature dehydration method; D: Glycocalyx depth covering the podocyte quantified in electron microscopic images from healthy mice in three different dehydration methods eGLX: endothelial glycocalyx; GBM: glomerular basement membrane; pGLX: podocyte glycocalyx.
2.7. Statistical analysis
Statistical analyses were performed by GraphPad Prism version 9.0 (GraphPad software, United States). Each experiment was repeated three times and all the original data were showed as mean ± SEM. One-way analysis of variance and repeated-measures analysis of variance followed by the LSD multiple comparison test were used for comparisons of multiple groups. Values of p < 0.05 were considered statistically significant.
3. Results
3.1. Effects of different dehydration methods on the aortic endothelial cells glycocalyx structure in mice
The aorta of the same healthy mice was divided into three equal parts, one part was dehydrated by gradient with ethanol (Fig. 1A), the second part was dehydrated by gradient with acetone (Fig. 1B), the third part was dehydrated by low temperature dehydration method (Fig. 1C), and the other processing steps were the same. The transmission electron microscope picture is shown in Fig. 1. The results showed that the mice aortic endothelial cells dehydrated by ethanol (Fig. 1A) had a layer of glycocalyx attached to the outside, the distribution was sparse, and the structure was loose; the mice aortic endothelial cells dehydrated with acetone (Fig. 1B) had a layer of glycocalyx attached to the outside, the glycocalyx layer is clear, well-preserved, and closely arranged. It can be seen that the glycocalyx layer extending outward presents a needle-like structure. And the endothelial cells of the mice aorta dehydrated by the low temperature dehydration method (Fig. 1C) had a clear structure, plump shape, obvious organelles in the cell matrix, the glycocalyx layer was evenly distributed on the outside of the cell membrane, the preservation was relatively complete, and the glycocalyx has a certain thickness. After magnification, it can be seen that the glycocalyx presents a needle-like structure. In the mice aorta using the acetone gradient dehydration method (Fig. 1B), the endothelial cells structure is complete, the organelles in the cell matrix are blurred, and most of the glycocalyx layer outside the cell membrane was dropped (Fig. 1B), and the distribution is uneven. Scattered needle-like glycocalyx structures can be seen around. As shown in Fig. 1B, the glycocalyx layer is thin and difficult to distinguish and the needle-like structures are not obvious after magnification. However, for the aorta, low temperature dehydration and acetone gradient dehydration yielded greater glycocalyx depth than ethanol gradient dehydration (Fig. 1D). The endothelial glycocalyx depth in mice aorta was about 45 nm with conventional ethanol gradient dehydration, while the endothelial glycocalyx depth in mice aorta was nearly 60 nm with acetone gradient dehydration and low temperature dehydration (Fig. 1D). However, the “needle-like” structure of the glycocalyx of aorta in mice preserved by dehydration at low temperature was more obvious (Fig. 1C). And the coverage rate of aorta glycocalyx obtained by different dehydration methods were 100%. In conclusion, low temperature dehydration can better preserve the fine structure of glycocalyx in mice aorta.
3.2. Effects of different dehydration methods on the glomerular glycocalyx structure in mice
Mice kidney was dissected, and the corticomedulary junction was taken and divided into three parts for ultra-thin section preparation. One part was dehydrated by ethanol gradient (Fig. 2A), the second part was dehydrated by acetone gradient (Fig. 2B); and the third part was dehydrated by low temperature (Fig. C). Foot process glycocalyx locating the glomerular basement membrane was observed and captured by transmission electron microscope (Fig. 2). The results showed that most of the glycocalyx structures dehydrated by gradient dehydration with ethanol fell off, and the distribution was uneven. However, the glycocalyx dehydrated by gradient dehydration with acetone had uniform distribution and clear structure. Therefore, in the subsequent freezing replacement process, acetone was selected as the replacement reagent. Glycocalyx was partially dropped outside the podocyte of the mice glomerulus dehydrated with acetone gradient (Fig. 2B), but the glycocalyx layer and the needle-like structure of the glycocalyx could be clearly observed after magnification. Using the low temperature dehydration method, the glycocalyx outside the podocyte of the mice glomerulus (Fig. 2C) was mostly dropped, and the needle-like structure was not obvious after magnification. The glycocalyx depth obtained by the three dehydration methods were measured, and the results are shown in Fig. 2D. The three dehydration methods had no significant differences on the glycocalyx depth. For the kidney, low temperature dehydration was greater than the podocyte glycocalyx depth obtained by acetone gradient dehydration and ethanol gradient preparation. The podocyte glycocalyx depth in mice kidney was about 20 nm with conventional ethanol and acetone gradient dehydration, while the endothelial glycocalyx depth in mice aorta was nearly 30 nm with low temperature dehydration (Fig. 2D). However, the error bars for kidney glycocalyx depth obtained by low temperature dehydration were larger. This suggests that low temperature dehydration kidney podocyte glycocalyx samples is heterogeneous. Only partially long and complete glycocalyx was present (Fig. 2. C). In addition, the coverage rate of kidney glycocalyx obtained by different dehydration methods were 100%.
3.3. Effects of different dehydration methods on the HUVEC glycocalyx structure
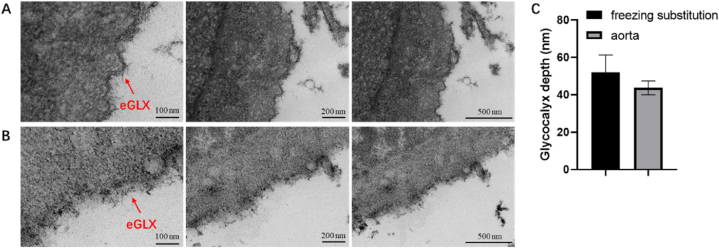
Based on the results of animal experiments, we further compared the effect of acetone gradient dehydration and low temperature dehydration on the preservation of endothelial glycocalyx structure in cellular level. Normally cultured HUVEC cells are divided into two parts, one part was dehydrated by low temperature dehydration method (Fig. 3A), the second part was dehydrated by gradient with acetone (Fig. 3B), and the other processing steps were the same. The glycocalyx structure of HUVEC obtained by gradient dehydration with acetone was not clear, while the glycocalyx structure of HUVEC obtained by low temperature dehydration showed obvious needle-like structure. The glycocalyx depths obtained by the two dehydration methods were measured, and the results are shown in Fig. 3C. The results indicated that the two dehydration methods had no significant differences on the glycocalyx depth. However, for the HUVEC, the glycocalyx depth was greater in low temperature dehydration method than those in acetone gradient dehydration method. The HUVEC glycocalyx depth was about 50 nm with low temperature dehydration, but glycocalyx depth was only 40 nm deep in acetone gradient dehydration method (Fig. 3C). Therefore, the results indicated low temperature dehydration can better preserve the glycocalyx structure of HUVEC. In addition, the coverage rate of HUVEC glycocalyx obtained by different dehydration methods was 100%.
Fig. 3.
Transmission electron microscope images of HUVEC glycocalyx. A: Using low temperature dehydration method; B: Using acetone gradient dehydration method; C: Glycocalyx depth covering the HUVEC quantified in electron microscopic images in different dehydration methods. eGLX: endothelial glycocalyx.
3.4. Immunofluorescence images of glycocalyx
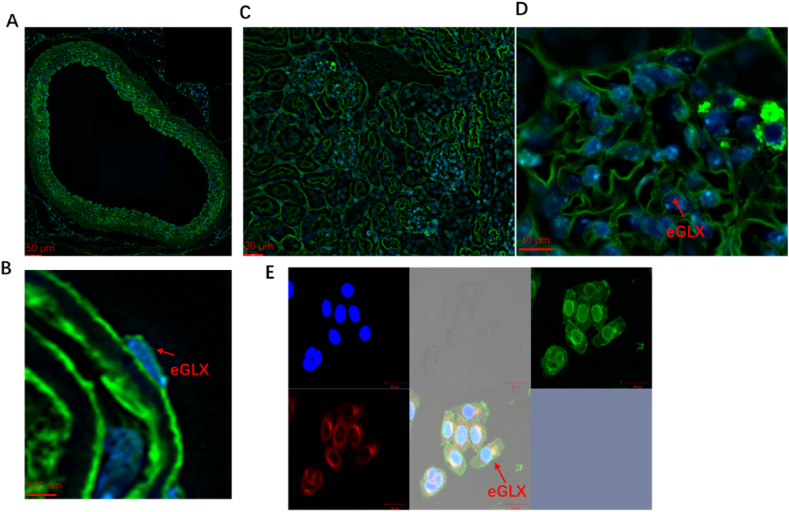
Currently, WGA (Wheat Germ Agglutinin) is commonly used as a marker for glycocalyx. Glycocalyx was obtained by conventional chemical fixation/ethanol gradient dehydration (Fig. 4). The outermost green color represents the labeled glycocalyx layer. The results showed that normal HUVEC (Fig. 4 E), mouse kidney (Fig. 4C–D) and aorta (Fig. 4A–B) all had complete and clear glycocalyx layer.
Fig. 4.
Immunofluorescence images of glycocalyx. A, B: Immunofluorescence images of mice aorta glycocalyx; Fig. 4B is a higher magnification of 4 A. C, D: Immunofluorescence images of mice kidney glycocalyx; Fig. 4D is a higher magnification of 4.C. E: Immunofluorescence images of HUVEC glycocalyx. Green: WGA (Wheat Germ Agglutinin), blue: DAPI, red: Dil. eGLX: endothelial glycocalyx. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
Glycocalyx is located outside the vascular endothelial cells playing an important role in vascular homeostasis. The complete preservation of glycocalyx in vitro and the optimization sample preparation technology are very important for study glycocalyx and glycocalyx related disease. At present, there are various methods to observe the glycocalyx structure, but they all have limitations [6]. Ruthenium red staining combined with glutaraldehyde/osmic acid fixation is a classical method used in early TEM [38]. However, this method has its limitations: on the one hand, ruthenium red is a macromolecular substance, and it cannot completely enter the entire glycocalyx layer to make it colored; On the other hand, the electrostatic interaction between ruthenium red on the surface of the cell membrane and the glycocalyx side chain can change the spatial geometry of the glycocalyx [39]. Haldenby et al. [40] studied the small molecular weight Achine blue (alcianblue) instead of ruthenium red was used to study the thickness of glycocalyx in rabbit arterial system. The measurements ranged from 45 nm to 80 nm, and the size of the glycocalyx in the thoracic aorta was about 60 nm. Chappell et al. [41] compared the TEM results of glycocalyx on the surface of umbilical vein endothelial cells under perfusion fixed and immersing-fixed methods, and found that the presence of glycocalyx could not be observed in TEM images obtained under immersion fixation. In contrast, perfusion fixation has more advantages. Fluorescence microscopy has optical limitations, and it is far from sufficient to fully demonstrate the geometrical characteristics of glycocalyx [42]. The measurement of glycocalyx size by side-flow dark field imaging is limited to the microcirculation system [43]. Due to the superiority of resolution, transmission electron microscopy plays an important role in the study of glycocalyx. However, in the process of conventional fixation and dehydration, the glycocalyx structure would inevitably be destroyed. Therefore, it is necessary to explore a more suitable method for the observation of glycocalyx.
At present, chemical fixation/ethanol gradient dehydration is usually used for the preservation of glycocalyx. It can be seen from Fig. 1, Fig. 2 that for the aorta and kidney glycocalyx in mice, under the same conditions as other treatments, the glycocalyx preservation by acetone gradient dehydration is better than that of ethanol gradient dehydration. The reason may be that ethanol is more polar than acetone, so ethanol causes tissue water content decrease too quickly, which is not conducive to the preservation of fine structure. Acetone dehydration is gentler and can better preserve fine structures such as glycocalyx. Also acetone dehydration steps are less than ethanol dehydration, and acetone is miscible with resin. This enables acetone dehydration better preserve the glycocalyx structure. For the preservation of the aorta glycocalyx, the low temperature dehydration method can better preserve the villi-like glycocalyx structure. The glycocalyx has a complete shape, a clear structure, and can clearly reflect the details of the glycocalyx structure. Comparing the electron microscope results of the other two dehydration methods, the low temperature dehydration method can retain the glycocalyx structure to a great extent, reduce the deformation, and make the structure clear, reflect the glycocalyx ultrastructure more truly. This may be because low temperature dehydration can better retain the glycocalyx tissue moisture and reduce damage to the glycocalyx structure during the sample preparation process.
However, low temperature dehydration is not ideal for the preservation of the glycocalyx structure in mice kidney samples, which may be due to low temperature dehydration requires smaller sample size. The large size of the kidney tissue and the insufficient penetration of organic solvents during the low temperature dehydration process increase the sample preparation difficulty. Thus the preservation of the glycocalyx is incomplete and affects the observations [44]. Therefore, when using low temperature dehydration for kidney glycocalyx sample preparation, it can be considered to cut the tissue block smaller to facilitate penetration. In addition, while aldehydes such as formaldehyde or glutaraldehyde preserve the structure of the sample, they would cause cross-linking between the glycocalyx various components, which has a certain compression effect on the glycocalyx and distorts the glycocalyx structure [32,45]. E.E. Ebong et al. [32] obtained images of the thickness of the glycocalyx on the surface of bovine thoracic aortic endothelial cells (BAEC) with a thickness of about 11 μm under the premise of preserving the hydration structure of the glycocalyx by rapid freezing replacement transmission electron microscopy. This suggests that high-pressure freezing/freezing substitution are worthy consideration for the glycocalyx samples preparation. However, high-pressure freezing/freezing substitution also have disadvantages. The pressure damage in physical fixation and the requirement for the thickness of the sample will cause the sample to crack, break or lose [46]. In addition, high-pressure freezing/freezing substitution requires on-site sampling, high requirements for sample preparation, and expensive and less instruments, which is not conducive to many experiments be conducted. However, we adopt the chemical fixation-freezing method, which reduces the requirements for material collection and sample preparation. As the samples are fixed, they can be stored and mailed, which greatly saves costs and resources, and makes the experiment more universal. Also, the three methods of dehydration did not affect the glycocalyx depth (Fig. 1, Fig. 2, Fig. 3, Fig. 4) and coverage (Fig. 1, Fig. 2, Fig. 3, Fig. 4) in healthy mice, but the method of low temperature dehydration could better preserve the fine structure of the glycocalyx (Fig. 1, Fig. 2, Fig. 3). This may be because the glycosaminoglycans that make up the glycocalyx will not be decomposed and dropped due to the water content decrease, but the structure will not collapse due to the rapid water content decrease during the dehydration process. In addition, in the study of Sophie Dogné et al. [27], the thickness of the myocardial arterioles glycocalyx in healthy mice was approximately 40 nm; In our experiment, the thickness of the aortic glycocalyx of healthy mice is about 50 nm by using low temperature dehydration method. And Raina D. Ramnath et al. [47] and Sebastian Oltean et al. [28] found that healthy mice kidney glycocalyx depth is about 25 nm. In our experiment, when we used low temperature dehydration method treating mice kindy, we also find the thickness of the kindy glycocalyx of healthy mice is about 25 nm. Therefore, low temperature dehydration method is worth to consider used for mice aorta glycocalyx preservation.
According to our TEM results, the glycocalyx coverage of HUVEC/kidney/aorta was 100% (Fig. 1, Fig. 2, Fig. 3). We used WGA to label the glycocalyx of HUVEC, mouse kidney/aorta, and although we used the traditional method of ethanol dehydration, the glycocalyx basis was left intact with 100% coverage. These results demonstrated complete glycocalyx coverage on a larger scale (Fig. 4). This indicated that dehydration mode did not affect the coverage of glycocalyx, but affected the depth and fine structure of glycocalyx. Preservation of fine structure of glycocalyx is the biggest advantage of low temperature dehydration over ethanol gradient dehydration and acetone gradient dehydration.
Therefore, we believe that low temperature dehydration is a good choice for TEM sample preparation for the large blood vessel glycocalyx represented by the aorta glycocalyx and cells cultured in vitro. In the microvascular glycocalyx represented by the renal glycocalyx, in this experiment, the dehydration method replaced by acetone was better. However, if the tissue can be better penetrated, low temperature dehydration is also worth considering.
5. Conclusion
Mice aorta and kidney glycocalyx was pre-chemical fixated by lanthanum nitrate staining, dehydrated through different methods such as ethanol gradient, acetone gradient and low temperature dehydration. The experimental results show that, for aorta glycocalyx, the sample preparation by low temperature dehydration is better than acetone gradient dehydration and ethanol gradient dehydration. For kidney glycocalyx, acetone gradient dehydration is better than ethanol gradient dehydration and low temperature dehydration. For the in vitro glycocalyx represented by HUVEC, the low temperature dehydration method was superior to acetone gradient dehydration and ethanol gradient dehydration. In a word, low temperature dehydration method is suitable for macrovascular glycocalyx (such as aortic glycocalyx) and in vitro cell (HUVEC) preservation and acetone gradient dehydration method is more suitable for microvascular glycocalyx (such as kidney glycocalyx) preservation. In addition, our study provided an optional, practical, convenient and convenient method for preparing glycocalyx samples, which greatly saved costs and resources, and makes the experiment more universal.
Author contribution statement
Zhi Li: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Quanbin Zhang; Ning Wu: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Yuan-yuan Sun: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
Quanbin Zhang was supported by Innovative Research Group Project of the National Natural Science Foundation of China {42176137}
Dr Ning Wu was supported by Nantong Science and Technology Project, China {MS12021037}
Data availability statement
Data included in article/supp. Material/referenced in article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The authors declare the following financial interests/personal relationships which may be considered as potential competing interests.
Acknowledgements
We would like to thank the Prof. Li Wang, Zhongshuang Lv, Bingxuan Huangfu of Center for Biological Imaging (CBI), Institute of Biophysics, Chinese Academy of Science for kindly help us conducted the Transmission electron microscope work.
Contributor Information
Yuan-yuan Sun, Email: sunyuanyuan@qdio.ac.cn.
Ning Wu, Email: wuning@qdio.ac.cn.
References
- 1.Chien S., Li S., Shyy J.Y.J. Effects of mechanical forces on signal transduction and gene expression in endothelial cells. Hypertension. 1998;31:162–169. doi: 10.1161/01.hyp.31.1.162. [DOI] [PubMed] [Google Scholar]
- 2.Luscher T.F., Barton M. Biology of the endothelium. Clin. Cardiol. 1997;20(II):3–10. [PubMed] [Google Scholar]
- 3.Cruz-Chu E.R., Malafeev A., Pajarskas T., Pivkin I.V., Koumoutsakos P. Structure and response to flow of the glycocalyx layer. Biophys. J. 2014;106:232–243. doi: 10.1016/j.bpj.2013.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang X., et al. Endothelial cell dysfunction and glycocalyx - a vicious circle. Matrix Biol. 2018;71–72:421–431. doi: 10.1016/j.matbio.2018.01.026. [DOI] [PubMed] [Google Scholar]
- 5.Squire J.M., et al. Quasi-periodic substructure in the microvessel endothelial glycocalyx: a possible explanation for molecular filtering? J. Struct. Biol. 2001;136:239–255. doi: 10.1006/jsbi.2002.4441. [DOI] [PubMed] [Google Scholar]
- 6.Reitsma S., Slaaf D.W., Vink H., van Zandvoort M., Egbrink M. The endothelial glycocalyx: composition, functions, and visualization. Pflueg. Arch. Eur. J. Physiol. 2007;454:345–359. doi: 10.1007/s00424-007-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao L., Lipowsky H.H. Composition of the endothelial glycocalyx and its relation to its thickness and diffusion of small solutes. Microvasc. Res. 2010;80:394–401. doi: 10.1016/j.mvr.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esko J.D., Selleck S.B. Order out of chaos: assembly of ligand binding sites in heparan sulfate. Annu. Rev. Biochem. 2002;71:435–471. doi: 10.1146/annurev.biochem.71.110601.135458. [DOI] [PubMed] [Google Scholar]
- 9.Funderburgh J.L. Keratan sulfate: structure, biosynthesis, and function. Glycobiology. 2000;10:951–958. doi: 10.1093/glycob/10.10.951. [DOI] [PubMed] [Google Scholar]
- 10.Laurent T.C., Fraser J.R.E. Hyaluronan. Faseb. J. 1992;6:2397–2404. [PubMed] [Google Scholar]
- 11.Lee J.Y., Spicer A.P. Hyaluronan: a multifunctional, megaDalton, stealth molecule. Curr. Opin. Cell Biol. 2000;12:581–586. doi: 10.1016/s0955-0674(00)00135-6. [DOI] [PubMed] [Google Scholar]
- 12.Sugahara K., et al. Recent advances in the structural biology of chondroitin sulfate and dermatan sulfate. Curr. Opin. Struct. Biol. 2003;13:612–620. doi: 10.1016/j.sbi.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Sieve I., Muenster-Kuehnel A.K., Hilfiker-Kleiner D. Regulation and function of endothelial glycocalyx layer in vascular diseases. Vasc. Pharmacol. 2018;100:26–33. doi: 10.1016/j.vph.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Garsen M., Rops A., Rabelink T.J., Berden J.H.M., van der Vlag J. The role of heparanase and the endothelial glycocalyx in the development of proteinuria. Nephrol. Dial. Transplant. 2014;29:49–55. doi: 10.1093/ndt/gft410. [DOI] [PubMed] [Google Scholar]
- 15.Satchell S.C., Braet F. Glomerular endothelial cell fenestrations: an integral component of the glomerular filtration barrier. Am. J. Physiol. Ren. Physiol. 2009;296:F947–F956. doi: 10.1152/ajprenal.90601.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haraldsson B., Nystrom J. The glomerular endothelium: new insights on function and structure. Curr. Opin. Nephrol. Hypertens. 2012;21:258–263. doi: 10.1097/MNH.0b013e3283522e7a. [DOI] [PubMed] [Google Scholar]
- 17.Singh A., et al. High glucose causes dysfunction of the human glomerular endothelial glycocalyx. Am. J. Physiol. Ren. Physiol. 2011;300:F40–F48. doi: 10.1152/ajprenal.00103.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Z., Wu N., Wang J., Zhang Q.B. Roles of endovascular calyx related enzymes in endothelial dysfunction and diabetic vascular complications. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.590614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eskens B.J.M., Zuurbier C.J., van Haare J., Vink H., van Teeffelen J.W.G.E. Effects of two weeks of metformin treatment on whole-body glycocalyx barrier properties in db/db mice. Cardiovasc. Diabetol. 2013;12 doi: 10.1186/1475-2840-12-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cochain C., Zernecke A. Macrophages in vascular inflammation and atherosclerosis. Pflueg. Arch. Eur. J. Physiol. 2017;469:485–499. doi: 10.1007/s00424-017-1941-y. [DOI] [PubMed] [Google Scholar]
- 21.Uchimido R., Schmidt E.P., Shapiro N.I. The glycocalyx: a novel diagnostic and therapeutic target in sepsis. Crit. Care. 2019;23 doi: 10.1186/s13054-018-2292-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nieuwdorp M., et al. Loss of endothelial glycocalyx during acute hyperglycemia coincides with endothelial dysfunction and coagulation activation in vivo. Diabetes. 2006;55:480–486. doi: 10.2337/diabetes.55.02.06.db05-1103. [DOI] [PubMed] [Google Scholar]
- 23.Mochizuki S., et al. Role of hyaluronic acid glycosaminoglycans in shear-induced endothelium-derived nitric oxide release. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H722–H726. doi: 10.1152/ajpheart.00691.2002. [DOI] [PubMed] [Google Scholar]
- 24.Chelazzi C., Villa G., Mancinelli P., De Gaudio A.R., Adembri C. Glycocalyx and sepsis-induced alterations in vascular permeability. Crit. Care. 2015;19 doi: 10.1186/s13054-015-0741-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thi M.M., Tarbell J.M., Weinbaum S., Spray D.C. The role of the glycocalyx in reorganization of the actin cytoskeleton under fluid shear stress: a "bumper-car" model. Proc. Natl. Acad. Sci. U.S.A. 2004;101:16483–16488. doi: 10.1073/pnas.0407474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luft J.H. Fine structure of capillary and endocapillary layer as revealed by ruthenium red. Fed. Proc. 1966;25:1773. [PubMed] [Google Scholar]
- 27.Dogne S., et al. Hyaluronidase 1 deficiency preserves endothelial function and glycocalyx integrity in early streptozotocin-induced diabetes. Diabetes. 2016;65:2742–2753. doi: 10.2337/db15-1662. [DOI] [PubMed] [Google Scholar]
- 28.Oltean S., et al. Vascular endothelial growth factor-A(165)b is protective and restores endothelial glycocalyx in diabetic nephropathy. J. Am. Soc. Nephrol. 2015;26:1889–1904. doi: 10.1681/ASN.2014040350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zengbo L. Second Military Medical University; 2014. The Role and Mechanism of Heparanase in Acute Kidney Injury in Sepsis. [Google Scholar]
- 30.Barua D., Nagel M., Winklbauer R. Cell-cell contact landscapes in Xenopus gastrula tissues. Proc. Natl. Acad. Sci. U.S.A. 2021;118 doi: 10.1073/pnas.2107953118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vink H., Duling B.R. Identification of distinct luminal domains for macromolecules, erythrocytes, and leukocytes within mammalian capillaries. Circ. Res. 1996;79:581–589. doi: 10.1161/01.res.79.3.581. [DOI] [PubMed] [Google Scholar]
- 32.Ebong E.E., Macaluso F.P., Spray D.C., Tarbell J.M. Imaging the endothelial glycocalyx in vitro by rapid freezing/freeze substitution transmission electron microscopy, arterioscler. Thromb. Vasc. Biol. 2011;31:1908–1915. doi: 10.1161/ATVBAHA.111.225268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vonschack M.L., Fakan S., Villiger W., Muller M. Cryofixation and cryosubstitution - a useful alternative in the analyses of cellular fine-structure. Eur. J. Histochem. 1993;37:5–18. [PubMed] [Google Scholar]
- 34.Bhat S.N., Sharma A., Bhat S.V. Vitrification and glass transition of water: insights from spin probe ESR. Phys. Rev. Lett. 2005;95 doi: 10.1103/PhysRevLett.95.235702. [DOI] [PubMed] [Google Scholar]
- 35.Sun X., Yang Z., Mu X., Zhang T. Effects of different fixatives on staining patterns of four lectins in cultured microvascular endothelial cells. J. Histotechnol. 2015;38:22–27. [Google Scholar]
- 36.Kang H., et al. Regional specific adaptation of the endothelial glycocalyx dimension in tail-suspended rats. Pflueg. Arch. Eur. J. Physiol. 2015;467:1291–1301. doi: 10.1007/s00424-014-1568-1. [DOI] [PubMed] [Google Scholar]
- 37.Wang G.-h., et al. Platelet microparticles contribute to aortic vascular endothelial injury in diabetes via the mTORC1 pathway. Acta Pharmacol. Sin. 2019;40:468–476. doi: 10.1038/s41401-018-0186-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janczyk P., Hansen S., Bahramsoltani M., Plendl J. The glycocalyx of human, bovine and murine microvascular endothelial cells cultured in vitro. J. Electron. Microsc. 2010;59:291–298. doi: 10.1093/jmicro/dfq007. [DOI] [PubMed] [Google Scholar]
- 39.Pries A.R., Secomb T.W., Gaehtgens P. The endothelial surface layer. Pflueg. Arch. Eur. J. Physiol. 2000;440:653–666. doi: 10.1007/s004240000307. [DOI] [PubMed] [Google Scholar]
- 40.Haldenby K.A., Chappell D.C., Winlove C.P., Parker K.M., Firth J.A. Focal and regional variations in the composition of the glycocalyx of large vessel endothelium. J. Vasc. Res. 1994;31:2–9. doi: 10.1159/000159025. [DOI] [PubMed] [Google Scholar]
- 41.Chappell D., et al. The glycocalyx of the human umbilical vein endothelial cell an impressive structure ex vivo but not in culture. Circ. Res. 2009;104:1313–1317. doi: 10.1161/CIRCRESAHA.108.187831. [DOI] [PubMed] [Google Scholar]
- 42.Gretz J.E., Duling B.R. Measurement uncertainties associated with the use of bright-field and fluorescence microscopy in the microcirculation. Microvasc. Res. 1995;49:134–140. doi: 10.1006/mvre.1995.1011. [DOI] [PubMed] [Google Scholar]
- 43.den Uil C.A., et al. The microcirculation in health and critical disease. Prog. Cardiovasc. Dis. 2008;51:161–170. doi: 10.1016/j.pcad.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 44.Ge L., et al. The application of highpressure freezing-freeze substitution technique in ultrastructure of different biological samples. J. Chin. Electron. Microsc. Soc. 2021;40:61–66. [Google Scholar]
- 45.Heuser J. Whatever happened to the 'microtrabecular concept'? Biol. Cell. 2002;94:561–596. doi: 10.1016/s0248-4900(02)00013-8. [DOI] [PubMed] [Google Scholar]
- 46.Zhang B., et al. The application of highpressure freezing-freeze substitution technique in ultrastructure of nervous tissue. J. Chin. Electron. Microsc. Soc. 2017;36:45–50. [Google Scholar]
- 47.Ramnath R.D., et al. Blocking matrix metalloproteinase-mediated syndecan-4 shedding restores the endothelial glycocalyx and glomerular fi ltration barrier function in early diabetic kidney disease. Kidney Int. 2020;97:951–965. doi: 10.1016/j.kint.2019.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. Material/referenced in article.