Abstract
Background
Opioid overdoses are a growing concern, particularly among people who inject drugs. Sweden, with a comparatively high proportion of drug-related mortality, introduced its first Take-Home Naloxone (THN) program in 2018, at the Stockholm needle and syringe program (NSP). In this study we compare THN participant characteristics regarding refills and overdose reversals as well as investigate predictors associated with number of reversals. We also investigate interventions performed in overdose situations and endpoints for naloxone doses.
Methods
This was a prospective open inclusion cohort study conducted between January 24th 2018 and March 31st 2022 at the Stockholm NSP. Participants received THN, free of charge, after a training session and provided data regarding drug use and overdose experiences. During refill visits, participants reported if the naloxone was used for overdose reversal and, if so, responded to a ten-item questionnaire which included stating whether the naloxone recipient was the participant themselves or somebody else. Questionnaire data was combined with NSP database demographic data. Zero-inflated Poisson regression was applied to analyse predictors for number of reported overdose reversals.
Results
Among study participants (n = 1,295), 66.5% stated opioids as their primary drug, and 61.4% and 81.0% had previous experience of a personal or witnessed overdose, respectively. Overall, 44.0% of participants reported a total of 1,625 overdose reversals and the victim was known to have survived in 95.6% of cases. Stimulant use (aIRR 1.26; 95% CI 1.01, 1.58), benzodiazepine use (aIRR 1.75; 95% CI 1.1, 2.78) and homelessness (aIRR 1.35; 95% CI 1.06, 1.73) were predictors associated with an increased number of reported overdose reversals. Mortality was higher among those who reported at least one overdose reversal (HR 3.4; 95% CI 2.2, 5.2).
Conclusions
An NSP’s existent framework can be utilised to effectively implement a THN program, provide basic training and reach numerous high-risk individuals. During the four-year study, THN participants reversed a sizeable number of potentially fatal overdoses, of which many were reported by participants whose primary drug was not opioids. Naloxone refill rate was high, indicating that participants were motivated to maintain a supply of naloxone in case of future overdose events.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13011-023-00533-2.
Keywords: Take-Home Naloxone, People who inject drugs, Needle and syringe program, Opioid overdose, Sweden
Background
The number of opioid-related overdoses among people who use drugs (PWUD) has increased significantly in recent years, with more than 100,000 fatal opioid overdoses estimated in 2021 in the United States (U.S.) alone [1]. The use of fentanyl and other synthetic opioids has driven the opioid crisis in the U.S. and Canada [2] while heroin remains the most common illicit opioid used in Europe [3].
Drug-related mortality in Sweden rose in the mid-2000s, with opioid overdoses being the most common cause of death [4]. In 2018, the number of fatalities in Sweden decreased slightly, largely due to the reduced availability and use of fentanyl [4]. Despite this, Sweden had the highest proportion of drug-induced deaths in the European Union in 2018 with an estimated 84 deaths per million among adults [3].
While fatal opioid overdoses are the leading cause of death among PWUD, non-fatal overdoses are even more prevalent, especially among people who inject opioids [5, 6]. Non-fatal overdoses are costly for health care systems and can have serious consequences for victims, including hypoxic brain injury with cognitive impairment and memory loss, cardiac failure, peripheral neuropathy and pneumonia [7]. A non-fatal overdose is also a predictor for subsequent fatal drug overdose [6, 8, 9].
Naloxone is an opioid-specific antagonist that efficiently, but temporarily, reverses the acute effects of an opioid overdose thereby preventing potential injuries or fatal outcomes. Naloxone has no potential for abuse and reports of serious adverse effects are scarce, although opioid dependent individuals may experience unpleasant withdrawal symptoms [10]. A significant proportion of PWUD have both witnessed and experienced opioid overdoses at some point in life [11, 12] and in heroin related overdoses most fatalities occur 20–30 min after use, providing a window for naloxone interventions [13].
Naloxone was initially used as an emergency treatment for opioid overdose by health care personnel, but increased overdose numbers led to the initiation of the first Take-Home Naloxone (THN) programs in the U.S. in 1996 [14]. THN programs distribute naloxone directly to individuals at risk of experiencing or witnessing an opioid overdose, with the aim of a prompt initiation of overdose reversal in order to reduce mortality and morbidity. Short training sessions have proven to be sufficient to educate lay persons, significantly improving their knowledge of overdose management and safe naloxone administration [15–17]. Broad implementation of THN programs [18] as well as opioid agonist treatment (OAT) [19] and safer drug consumption sites [20] have been identified as key strategies that can reduce the number of fatal opioid overdoses.
Previous quantitative studies on THN programs have included cost effectiveness [21], level of training needed [15] and characteristics associated with naloxone refill and overdose reversals [22, 23]. A recent Swedish study compared the uptake and use of THN in OAT and needle and syringe program (NSP) populations and concluded that NSP clients constituted a high-risk group that was more likely to report overdose reversals [24]. Other Nordic research has focused on the role of “super users”, i.e. people who have reported more than three overdose reversals, highlighting young age, heroin use and prior overdose experience as significant characteristics of this population [25].
In Sweden, it took several years to overcome regulations that hampered the introduction of THN programs and when the National Board of Health and Welfare finally approved THN implementation in June 2017, naloxone was not made available for THN programs until the following year. While widespread in some parts of the country [26], THN cover is patchy elsewhere [27].
Policy and legal barriers continue to prevent THN programs from fulfilling their full potential to save lives [14]. Sweden lacks a national government-funded THN program, something which its neighbour Norway has had since 2014 [28]. According to Swedish legislation, THN prescription is personal and restricted to individuals at risk of an overdose, it must also be combined with information and basic training. Consequently, the family or friends of a person at risk cannot be prescribed naloxone [29], however the actual overdose training material can be accessed online by family members and other potential bystanders [30], or via some regional initiatives [26]. Considering these limitations, current THN programs need to be assessed in order to further inform health care decisions and policy makers.
In this study we use data from Sweden’s first official THN program which was introduced at the Stockholm NSP in 2018. The study sample consists of people who inject drugs (PWID), a high-risk group per se, in a setting where a high prevalence of opioid overdoses was anticipated. The aim of the study was to compare THN participant characteristics regarding naloxone refill and reports of overdose reversal and to further investigate:
Predictors associated with the number of reports of overdose reversal among THN participants.
Overdose situations and interventions performed, when THN was used.
Naloxone endpoints i.e. what happened to the individual naloxon doses given out in the THN program.
Methods
Study setting
The Stockholm NSP opened in April 2013 and consists of two clinics and one mobile unit. To date, more than 4,300 individuals have been registered in the program. The NSP offers sterile injection equipment such as needles, syringes and other paraphernalia (filters and cookers). In addition, services include THN and overdose prevention training, vaccination, counselling, wound care, treatment for hepatitis C (HCV) and HIV as well as referrals to other service providers for OAT and other substance use disorder treatments. In accordance with Swedish legislation, eligibility criteria for NSP enrolment include current injection drug use, being 18 years of age or older, and being able to prove your identity. Testing for hepatitis A, hepatitis B, HCV and HIV is offered on enrolment and continuously. The unique Swedish personal identity number is used for registration and those without such an ID are provided with a unique reserve number.
Study inclusion criteria were THN program enrolment in the Stockholm NSP and at least one additional NSP visit during the study period.
Study design and THN intervention
This was a prospective open inclusion cohort study conducted between January 24th 2018 and March 31st 2022. Clients in the Stockholm NSP were informed and recruited to the THN program through personal information from NSP staff, also promoted on an information screen in the waiting room. The study design relied on the existing NSP structure with passive follow-up when participants returned to the NSP for regular visits, there was no active following-up outside of the NSP. During the study period’s first four months, the only available naloxone on the Swedish market was a prefilled vial for intra muscular (i.m.) administration (Prenoxad, 0.4 mg/ml) which contained five doses of naloxone. From May 2018, i.m. naloxone was gradually replaced within a few months by an intranasal solution (Nyxoid, 1.8 mg/dose) which was given out in a kit containing two doses.
All THN participants individually completed a single training session, carried out by a NSP nurse or physician, before naloxone was handed out the first time. The sessions were brief (10–15 min) and conducted on the premises of the NSP clinics. A refresher training session was offered on-demand when returning for a refill of naloxone. The training protocol was based on the European Monitoring Centre for Drugs and Drug Addiction’s (EMCDDA) recommendations [31] and included information on how to identify an overdose, general overdose response, basic cardiopulmonary resuscitation (CPR) with focus on rescue breathing, and how to administer the naloxone (i.m. or nasal). Participants were informed of possible side effects, including the risk of withdrawal symptoms, the half-life of naloxone and the importance of calling for an ambulance in an overdose situation. At the end of the session, participants were given the opportunity to practice their skills on a CPR dummy and received a prefilled vial of naloxone for i.m. use, or two doses of nasal naloxone, free of charge. Participants were advised to return for a refill and debriefing if their current dose was used for an overdose reversal, lost, expired, given away or in any other way went missing. Participants did not receive any renumeration for participation in study.
THN participants were offered a pocket-sized brochure with key messages from the training. One year into the project, the National Board of Health and Welfare published standardised material concerning THN programs and overdose prevention, which was then distributed in the training sessions [30].
Questionnaires and definitions of measurements
All study data was registered in the national quality register InfCare Needle Syringe Program (InfCare NSP) database, previously described in detail [32]. Six InfCare NSP questionnaires were used for this study: 1) the ‘NSP enrolment questionnaire’: basic socio-demographic data and drug history; 2) the ‘NSP standard visit questionnaire’: at every NSP visit, clients report which drug they last injected; 3) the ‘Three-to-six-month NSP follow up questionnaire’: updated information on employment and housing status; 4) the ‘Twelve-month NSP follow-up questionnaire’: updated information on employment, housing status and primary drug the past 12 months; 5) the ‘THN enrolment questionnaire’: primary drug and previous experiences of drug overdose; and 6) the ‘THN refill questionnaire’: information on what happened to the previous naloxone dose and, if naloxone was used to reverse an overdose, ten follow-up questions on the intervention.
THN questions were partly adapted from the Norwegian THN training curriculum [33].
Overdose reversals were reported by the person whose prescribed naloxone dose had been used in the event. Consequently, the person experiencing the overdose could have been the study participant themselves or somebody else.
The latest available information was presented for variables concerning housing, education level, income type and primary drug. Static baseline variables included: age at inclusion in THN program, gender, country of birth, and experience of personal or witnessed overdose at inclusion in THN program. Housing status was re-coded into three main categories: homeless (which included sleeping in tents, in garages, in night shelters, on friends’ sofas and so on); unstable housing (temporary housing like hostels, rehabilitation homes etc.) and stable housing (longer-term rental or own home).
For intramuscular naloxone (which was only distributed in the first few months of the program), although the vial technically contained five doses, it was treated as a single dose in reporting.
Data on mortality were automatically reported from the Swedish population registry to the Stockholm NSP medical chart and quality register.
Statistics
Continuous variables were presented both as mean (standard deviation) and median (inter quartile range) values, and categorical variables as counts and percentages. Between-group differences were presented using risk ratios (RR) for categorical variables and mean and median differences for continuous variables. RR were estimated using a log-linear model and mean and median differences using linear and quantile regression respectively.
The association of baseline information and number of naloxone doses used at overdose was estimated in Incidence Rate Ratios (IRR) using a zero-inflated Poisson regression model, offsetting for the time participants spent in the study (time-in-study). The offset was applied due to the high variance of time-in-study which directly affected the probability of reporting overdose reversal. Univariable models as well as a multivariable model with all covariates were estimated. All variables from the unadjusted model were included in the adjusted model (gender, age, country of birth, housing, primary drug, and overdose experience). The logistic zero-inflation part of the model only used the number of naloxone doses received as an independent variable, while the independent variables in the Poisson part of the model were changed depending on the variable of interest.
The association between background information and the endpoint of individual naloxone doses (i.e. was it used, lost, stolen etc.) was estimated in Relative Risk Ratios (RRR) using a multinomial logistic regression model. The clustered robust standard error estimator was used to account for repeated measures since a person could report different endpoints multiple times due to receiving more than one refill.
The difference in mortality between the group of participants that had reported at least one overdose reversal compared to the group that had not, was estimated in Hazard Ratios (HR) using an illness-death model based on the Cox proportional hazards model.
Due to very low levels of missing data, we restricted the analysis to subjects with complete data on the variables involved. Confidence interval (CI) level was set at 95%. All reported p-values are two-sided and a p-value of < 0.05 was considered as statistically significant.
Analysis was done in Stata, version 15 (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC).
Results
Participants and characteristics
During the study period, 3,151 individuals visited the Stockholm NSP and 1,438 of them enrolled in the THN program. After excluding 143 people who did not make a return visit to the NSP during the study period, the final analysis contained 1,295 individuals. Naloxone was used in 1,625 separate overdose events, reported by 570 individuals, during the four-year study period (Fig. 1). A total of 11,440 doses of naloxone were distributed (2,590 at enrolment and 8,850 at subsequent refills).
Fig. 1.
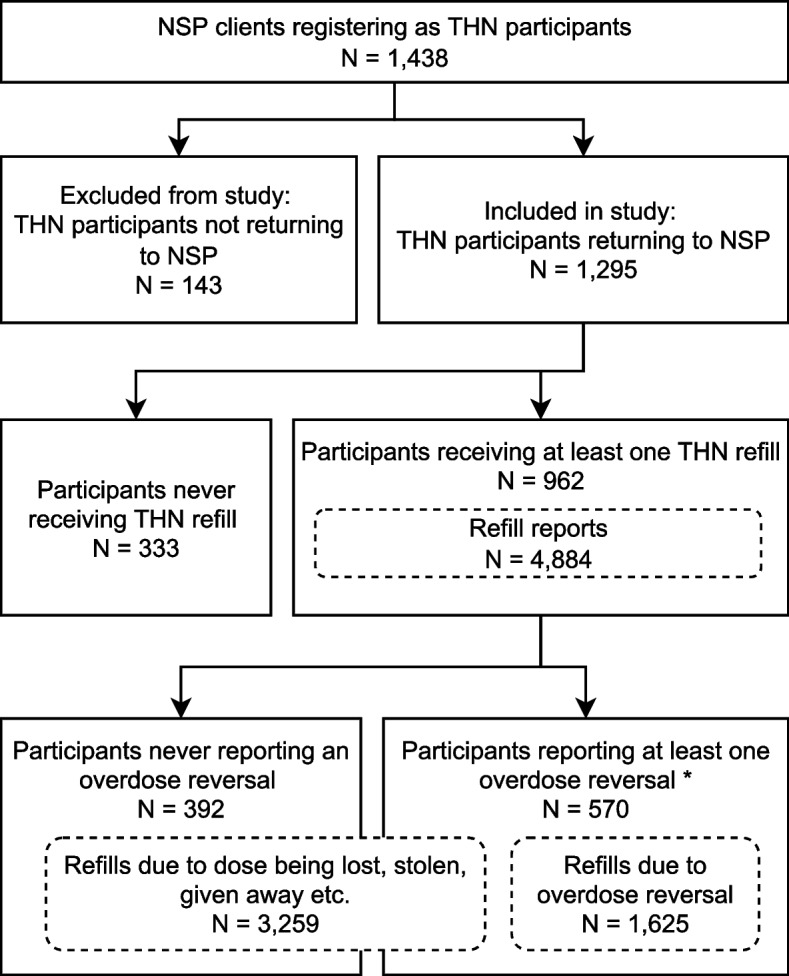
Flowchart of THN participants
* Since participants could make multiple refill reports, some of the reports they submitted were for doses not used for overdose reversal
The mean age of participants was 38, with the majority being male and born in Sweden, in line with the general population in the Stockholm NSP [32]. The majority stated opioids (mostly heroin) as their primary drug and had prior experience of personal as well as witnessed overdoses (Table 1).
Table 1.
Characteristics of Take-Home Naloxone participants by refill and reversal status (N = 1,295)
| Variable | All | No Refill | Refill received | RR (CI) | P-value | No Reversal | Reversal reported | RR (CI) | P-value |
|---|---|---|---|---|---|---|---|---|---|
| N = 1,295 | N = 333 | N = 962 | N = 392 | N = 570 | |||||
| Gender | N (%) | N (%) | N (%) | N (%) | N (%) | ||||
| Man | 951 (73.4) | 256 (76.9) | 695 (72.2) | 1 (ref) | - | 266 (67.9) | 429 (75.3) | 1 (ref) | - |
| Woman | 344 (26.6) | 77 (23.1) | 267 (27.8) | 1.06 (0.99, 1.14) | 0.09 | 126 (32.1) | 141 (24.7) | 0.86 (0.75, 0.97) | < .02 |
| Age at inclusion | |||||||||
| Mean (SD) | 38.0 (11.3) | 39.8 (11.8) | 37.4 (11.0) | -2.4 (-3.8, -1.01) | 0.001 | 37.9 (11.3) | 37.0 (10.7) | -0.87 (-2.29, 0.54) | 0.23 |
| Median (IQR) | 36 (29–46) | 39 (30–50) | 35.5 (29–45) | -3 (-5.19, -0.81) | < .01 | 36 (29–46) | 35 (29–44) | -1 (-2.96, 0.96) | 0.32 |
| Country of birth | |||||||||
| Sweden | 984 (76.0) | 248 (74.5) | 736 (76.5) | 1 (ref) | - | 305 (77.8) | 431 (75.6) | 1 (ref) | - |
| Rest of Europe | 109 (8.4) | 34 (10.2) | 75 (7.8) | 0.92 (0.81, 1.05) | 0,21 | 35 (8.9) | 40 (7.0) | 0.91 (0.73, 1.14) | 0.41 |
| Rest of the World | 150 (11.6) | 42 (12.6) | 108 (11.2) | 0.96 (0.87, 1.07) | 0.48 | 36 (9.2) | 72 (12.6) | 1.14 (0.98, 1.32) | 0.08 |
| Missing | 52 (4.0) | 9 (2.7) | 43 (4.5) | - | - | 16 (4.1) | 27 (4.7) | - | - |
| Housing situation | |||||||||
| Stable | 451 (34.8) | 139 (41.7) | 312 (32.4) | 1 (ref) | - | 132 (33.7) | 180 (31.6) | 1 (ref) | - |
| Unstable | 532 (41.1) | 124 (37.2) | 408 (42.4) | 1.11 (1.03, 1.20) | < .01 | 169 (43.1) | 239 (41.9) | 1.02 (0.90, 1.15) | 0.81 |
| Homeless | 238 (18.4) | 52 (15.6) | 186 (19.3) | 1.13 (1.03, 1.24) | < .01 | 69 (17.6) | 117 (20.5) | 1.09 (0.94, 1.26) | 0.25 |
| Other | 68 (5.3) | 17 (5.1) | 51 (5.3) | 1.08 (0.93, 1.26) | 0.29 | 20 (5.1) | 31 (5.4) | 1.05 (0.83, 1.34) | 0.67 |
| Missing | 6 (0.5) | 1 (0.3) | 5 (0.5) | - | 2 (0.5) | 3 (0.5) | - | - | |
| Income type | |||||||||
| Stable | 207 (16.0) | 57 (17.1) | 150 (15.6) | 1 (ref) | - | 66 (16.8) | 84 (14.7) | 1 (ref) | - |
| Irregular | 1,040 (80.3) | 262 (78.7) | 778 (80.9) | 1.03 (0.94, 1.13) | 0.49 | 311 (79.3) | 467 (81.9) | 1.07 (0.92, 1.25) | 0.37 |
| Other | 40 (3.1) | 12 (3.6) | 28 (2.9) | 0.97 (0.78, 1.20) | 0.76 | 12 (3.1) | 16 (2.8) | 1.02 (0.72, 1.45) | 0.91 |
| Missing | 8 (0.6) | 2 (0.6) | 6 (0.6) | - | - | 3 (0.8) | 3 (0.5) | - | - |
| Education level | |||||||||
| < 9 years | 138 (10.7) | 29 (8.7) | 109 (1.3) | 1 (ref) | - | 42 (10.7) | 67 (11.8) | 1 (ref) | - |
| 9 years | 554 (42.8) | 141 (42.3) | 413 (42.9) | 0.94 (0.85, 1.04) | 0.25 | 162 (41.3) | 251 (44.0) | 0.99 (0.84, 1.17) | 0.9 |
| > 9 years | 576 (44.5) | 156 (46.8) | 420 (43.7) | 0.92 (0.84, 1.02) | 0.12 | 182 (46.4) | 238 (41.8) | 0.92 (0.78, 1.09) | 0.35 |
| Missing | 27 (2.1) | 7 (2.1) | 20 (2.1) | - | - | 6 (1.5) | 14 (2.5) | - | - |
| Primary drug | |||||||||
| Opioids | 861 (66.5) | 206 (61.9) | 655 (68.1) | 1 (ref) | - | 255 (65.2) | 400 (70.3) | 1 (ref) | - |
| Stimulants | 372 (28.7) | 109 (32.7) | 263 (27.3) | 0.93 (0.86, 1.0) | 0.06 | 118 (30.2) | 145 (25.5) | 0.90 (0.80, 1.02) | 0.11 |
| Benzodiazepines | 34 (2.6) | 4 (1.2) | 30 (3.1) | 1.16 (1.02, 1.32) | 0.02 | 13 (3.3) | 17 (3.0) | 0.93 (0.67, 1.28) | 0.65 |
| Other | 23 (1.8) | 11 (3.3) | 12 (1.2) | 0.69 (0.46, 1.02) | 0.06 | 5 (1.3) | 7 (1.2) | 0.96 (0.59, 1.55) | 0.59 |
| Missing | 5 (0.4) | 3 (0.9) | 2 (0.2) | 1 (0.3) | 0 (0.0) | - | - | ||
| Ever experienced personal overdose | |||||||||
| No | 454 (35.1) | 134 (40.2) | 320 (33.3) | 1 (ref) | - | 168 (42.9) | 152 (26.7) | 1 (ref) | - |
| Yes | 795 (61.4) | 183 (55.0) | 612 (63.6) | 1.09 (1.02, 1.17) | < .02 | 213 (54.3) | 399 (70.0) | 1.37 (1.21, 1.56) | < .001 |
| Missing | 46 (3.6) | 16 (4.8) | 30 (3.1) | - | - | 11 (2.8) | 19 (3.3) | - | - |
| Most recent personal overdose | |||||||||
| < 12 months ago | 340 (42.8) | 69 (37.7) | 271 (44.3) | 1 (ref) | - | 85 (39.9) | 186 (46.6) | 1 (ref) | - |
| > 12 months ago | 442 (55.6) | 114 (62.3) | 328 (53.6) | 0.93 (0.86, 1.01) | 0.07 | 123 (57.7) | 205 (51.4) | 0.91 (0.81, 1.02) | 0.11 |
| Missing | 13 (1.6) | 0 (0.0) | 13 (2.1) | - | - | 5 (2.3) | 8 (2.0) | - | - |
| Ever witnessed overdose | |||||||||
| No | 210 (16.2) | 61 (18.3) | 149 (15.5) | 1 (ref) | - | 82 (20.9) | 67 (11.8) | 1 (ref) | - |
| Yes | 1,049 (81.0) | 261 (78.4) | 788 (81.9) | 1.06 (0.96, 1.16) | 0.23 | 301 (76.8) | 487 (85.4) | 1.37 (1.14, 1.66) | 0.001 |
| Missing | 36 (2.8) | 11 (3.3) | 25 (2.6) | - | - | 9 (2.3) | 16 (2.8) | - | - |
| Most recent witnessed overdose | |||||||||
| < 12 months ago | 562 (53.6) | 118 (45.2) | 444 (56.3) | 1 (ref) | - | 132 (43.9) | 312 (64.1) | 1 (ref) | - |
| > 12 months ago | 465 (44.3) | 136 (52.1) | 329 (41.8) | 0.90 (0.83, 0.96) | < .01 | 162 (53.8) | 167 (34.3) | 0.72 (0.64, 0.82) | < .001 |
| Missing | 22 (2.1) | 7 (2.7) | 15 (1.9) | - | - | 7 (2.3) | 8 (1.6) | - | - |
| Time in study | |||||||||
| Mean days (SD) | 637.7 (470.0) | 319.1 (344.7) | 747.9 (456.7) | 1.00 (1.00, 1.00) | < .001 | 639.0 (427.4) | 822.8 (461.5) | 1.00 (1.00, 1.00) | < .001 |
RR Risk Ratio, CI Confidence Interval
By the end of the four-year study period, the majority of participants (74.3%) had returned to the NSP for at least one refill of naloxone and 44.0% had reported that their naloxone dose had been used in at least one overdose situation. Participants receiving a refill were more likely to: be younger, be homeless or in unstable housing, use benzodiazepines as their primary drug, have previous experience of personal overdose, or have a longer time-in-study. Reporting at least one overdose reversal with naloxone during the study period was associated with previous experience of personal or witnessed overdose, being male, and longer time-in-study. The comparison between participants receiving a refill or not, as well as participants reporting reversal or not, is depicted in Table 1.
Overall, 8.6% of the participants reporting reversals died during the study period compared to 4.1% within the group of participants not reporting reversals. The Cox regression analysis showed that the mortality was significantly higher among those who reported a reversal (HR 3.4; CI 2.2, 5.2).
Predictions of naloxone and number of reversals
We used a zero-inflated Poisson regression to evaluate predictors of number of reported overdose reversals among participants who received at least one refill. In the adjusted model, we noted a greater average number of reported reversals by homeless participants (aIRR 1.35; CI 1.06, 1.73) compared to those with other housing situations. Those with benzodiazepines (aIRR 1.75; CI 1.1, 2.78) or stimulants (aIRR 1.26; CI 1.01, 1.58) as their primary drug reported a greater average number of reversals than those who primarily used other drugs. Additionally, participants who were born outside of Europe (aIRR 1.37; CI 1.06, 1.76) also reported a higher average number of reversals than those born in Europe (including Sweden). The result of the unadjusted and adjusted model is summarized in Table 2.
Table 2.
Unadjusted and adjusted Zero-inflated Poisson multivariate model predicting naloxone reversal count among participants obtaining refill (N = 962)
| Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|
| Variables | IRR | 95% CI | P-value | aIRR | 95% CI | P-value |
| Gender | ||||||
| Man | 1 (ref) | - | - | 1 (ref) | - | - |
| Woman | 1.08 | 0.88, 1.33 | 0.45 | 1.08 | 0.87, 1.33 | 0.33 |
| Age at inclusion | ||||||
| Mean | 1.0 | 0.99, 1.01 | 0.79 | 1.0 | 0.99, 1.01 | 0.61 |
| Country of birth | ||||||
| Sweden | 1 (ref) | - | - | 1 (ref) | - | - |
| Rest of Europe | 0.89 | 0.69, 1.15 | 0.37 | 0.94 | 0.72, 1.23 | 0.63 |
| Rest of the World | 1.26 | 0.99, 1.61 | 0.06 | 1.37 | 1.06, 1.76 | 0.02 |
| Housing situation | ||||||
| Stable | 1 (ref) | - | 1 (ref) | - | ||
| Unstable | 1.1 | 0.91, 1.33 | 0.32 | 1.1 | 0.89, 1.34 | 0.38 |
| Homeless | 1.34 | 1.05, 1.72 | 0.02 | 1.35 | 1.06, 1.73 | 0.02 |
| Other | 0.84 | 0.60, 1.18 | 0.32 | 0.89 | 0.64, 1.24 | 0.48 |
| Primary drug | ||||||
| Opioids | 1 (ref) | - | 1(ref) | - | ||
| Stimulants | 1.14 | 0.92, 1.42 | 0.24 | 1.26 | 1.01, 1.58 | 0.04 |
| Benzodiazepines | 1.46 | 0.89, 2.42 | 0.14 | 1.75 | 1.1, 2.78 | 0.03 |
| Other | 1.12 | 0.54, 2.35 | 0.76 | 1.38 | 0.72, 2.68 | 0.33 |
| Ever experienced personal overdose (baseline) | ||||||
| No | 1 (ref) | - | 1(ref) | - | ||
| Yes | 1.14 | 0.93, 1.39 | 0.2 | 1.19 | 0.97, 1.47 | 0.10 |
| Ever witnessed overdose (baseline) | ||||||
| No | 1 (ref) | 1(ref) | ||||
| Yes | 1.3 | 1.00, 1.69 | < 0.05 | 1.27 | 0.95, 1.71 | 0.10 |
IRR Incidence Rate Ratio, CI Confidence Interval
Overdose situations
In the case of overdose reversals, the majority of refills (63.2%) were requested within four weeks of the incident (Table 3). In most cases (67%), overdose reversals were reported to have been carried out on an acquaintance. Just over half (51.8%) of the overdoses took place in a private space (normally the participant’s or somebody else’s home) while 45.3% occurred in a public space (most frequently “outdoors”). Homeless participants had more than twice the risk of reporting that the reversal took place in a public space than participants who were not homeless (RR 2.42; CI 1.60, 3.67).
Table 3.
Characteristics of overdose situations where THN was used (N = 1,625)
| Variable | N (%) |
|---|---|
| Who was the recipient of naloxone? | |
| Me | 264 (16.2) |
| Stranger | 149 ( 9.2) |
| Partner/Spouse | 100 ( 6.2) |
| Friend/acquaintance | 1,089 (67.0) |
| Missing | 23 ( 1.4) |
| Number of naloxone doses administered at overdose | |
| 1 | 779 (47.9) |
| 2 | 663 (40.8) |
| > 2 | 71 (4.4) |
| Missing | 112 (6.9) |
| When did the overdose take place? | |
| Less than a week ago | 401 (25.4) |
| 1–4 weeks ago | 626 (39.6) |
| 1–3 months ago | 311 (19.7) |
| 4–6 months ago | 132 (8.4) |
| 7–12 months ago | 74 (4.7) |
| Over a year ago | 35 (2.2) |
| Where did the overdose take place? | |
| Private space | 802 (51.8) |
| Public space | 701 (45.3) |
| Shelter | 36 (2.3) |
| Other | 8 (0.5) |
| Was CPR given? | |
| No | 1,057 (65.0) |
| Yes | 568 (35.0) |
| Was an ambulance called? | |
| No | 868 (53.4) |
| Yes | 757 (46.6) |
| If an ambulance was called, was the naloxone recipient taken to hospital? | |
| No | 218 (28.8) |
| Yes | 423 (55.9) |
| Other | 16 (2.1) |
| Don’t know | 100 (13.2) |
| Missing | 3 (0.2) |
| What drugs were believed to be used prior to the overdose? (multiple answers possible) | |
| Opioids | 1,537 (94.6) |
| Stimulants | 97 (6.0) |
| Benzodiazepines | 565 (34.8) |
| Other | 128 (7.9) |
| Did the naloxone recipient survive? | |
| Yes | 1,539 (95.6) |
| No | 8 (0.5) |
| Unknown | 59 (3.7) |
| How comfortable do you feel administering naloxone? | |
| Very comfortable | 1,167 (73.4) |
| Quite comfortable | 314 (19.7) |
| Only slightly comfortable | 11 ( 0.7) |
| Not at all comfortable | 5 ( 0.3) |
| Don’t know | 18 ( 1.1) |
| Missing | 76 ( 4.8) |
Apart from administering naloxone, overdoses were responded to with actions promoted in the overdose training such as rescue breathing and/or heart compressions (35%) or calling an ambulance (46.6%) (Table 3). The vast majority of participants reported that opioids (on their own or in combination with another drug) were believed to have been used prior to the overdose (Table 3). Apart from opioids, benzodiazepines were the most common additional drugs involved, reported in 34.8% of cases.
Participants reported that the person who experienced an overdose was known to have survived in 95.6% of incidents. There were eight cases (0.5%) where the person who experienced an overdose could not be resuscitated and subsequently died. The outcome of the remaining cases was unknown. The vast majority (93.1%) of the participants responded that they felt very comfortable, or quite comfortable, using naloxone in overdose situations.
Naloxone dose endpoints
THN participants made a total of 4,884 refill reports, stating that the previous dose was used in an overdose situation in 33.3% of these reports. Other reasons for refill were: dose lost (32.9%), dose given away (15.5%), dose stolen (4.9%) or “other reason” (13.3%, most commonly the previous dose had expired or been confiscated by police or security guards). We used multinomial logistic regression to explore participant predictors for different naloxone dose endpoints (Supplementary Tables S1a-c). The risk of giving away the naloxone dose was higher among participants with stimulants as their primary drug (RRR 1.46; CI 1.07, 1.98) compared to those who primarily used other substances, and lower amongst those with prior experience of personal overdose (RRR 0.60; CI 0.50, 0.88) or witnessed overdose (RRR 0.66; CI 0.41, 0,85) compared to participants with no such experiences. Having the dose stolen was more likely amongst participants who were homeless (RRR 3.73; CI 2.06, 6.75) or in unstable housing (RRR 1.9; CI 1.03, 3.42) compared to people with other housing situations; those whose primary drug was stimulants (RRR 1.77; CI 1.14, 2.74) compared to those who mainly used other substances; women (RRR 1.70; CI 1.17, 2.47) compared to men; and participants with prior experience of witnessed overdose (RRR 2.18; CI 1.12, 4.23) compared to those without such experience. Participants with experience of personal overdose on the other hand, were less likely to report this outcome (RRR 0.51; CI 0.34, 0.76). Lastly, the risk of losing the naloxone dose was higher among those with unstable housing (RRR 1.32; CI 1.04, 1.68) and homelessness (RRR 1.91; CI 1.47, 2.48) compared to participants with other housing situations and lower amongst those with personal (RRR 0.64; CI 0.50, 0.81) and witnessed overdose experience (RRR 0.65; CI 0.45, 0.94) compared to those without such experiences.
Discussion
In this study we investigated the first Take-Home Naloxone program introduced in Sweden, presenting demographic and behavioural characteristics associated with naloxone refill and reporting reversals as well as overdose situations and endpoints for distributed naloxone kits. We noted that over two thirds of the participants returned to the NSP for a refill during the study period, a higher proportion than reported in previous research [24, 34–36].
Overall, 44% of participants reported their naloxone being used to reverse an overdose at least once during the four-year long study period, totalling 1,625 overdose reversals. This is a large proportion compared to some other studies with similar passive follow‐up designs, which stated lower figures of between 7 and 20% of PWUD accessing THN programs reporting at least one of their doses being used for reversal [22, 24, 34, 36–39]. While this comparison may be tempered by differences in settings (such as prevalence of fentanyl) and the duration of the studies, the high number of reported reversals in our study suggests a high-risk study population in the NSP, and that experiencing and witnessing non-fatal overdoses was strikingly prevalent for THN program participants in Stockholm.
The majority of participants requested a new dose within four weeks of having used naloxone in an overdose event, implying that participants were motivated to maintain their naloxone supply and a low threshold for naloxone refill at the NSP. Although naloxone carriage rate was not specifically investigated in this study, previous research emphasises the importance of generous access to new doses in order to increase the chance of having naloxone accessible in critic situations [40–42].
The comparison between participants who reported overdose reversals and those who did not generally confirmed findings from previous studies on THN programs: that being a man [34] and prior experience of personal or witnessed overdose [22–24, 34, 43, 44] are factors associated with reporting overdose reversals. An unexpected finding in our study was that participants who stated stimulants or benzodiazepines as their primary drug reported a significantly greater average number of overdose reversals compared to people using opioids. This supports the argument for widespread distribution of naloxone to PWID who may not see themselves primarily as opioid users, also highlighted by Rowe et al. [22].
The data on which drugs were taken immediately before the overdose were self-reported by participants, and no information was collected regarding contamination of drugs. Swedish healthcare systems should consider its own readiness for sudden changes to the drug market, informed by experiences in other countries such as the impact of synthetic opioids on the U.S. and Canada [2] and how contaminated benzodiazepines have fuelled an epidemic of drug related deaths in Scotland [45]. Sudden changes to the supply of illegal drugs in Sweden will require the ability to promptly scale-up naloxone distribution [46].
Although the level of overdose reversals in our study was high, the majority of naloxone refills were not related to naloxone having been used in overdose situations. People with an unstable housing situation may face challenges in storing personal belongings, including naloxone, and as a result they had an increased risk of their dose being lost or stolen. Additionally, women were more likely than men to have their naloxone dose stolen, supporting previous research in the Stockholm NSP highlighting women’s vulnerability among PWID [47].
A current major challenge for increased access to naloxone in Sweden is that the national legislation is incompatible with prescribing naloxone to anyone other than the person at risk of overdosing [29]. In our study, 15.4% of the refills were due to the naloxone being given away to friends and family members, which pinpoints an unmet need for naloxone distribution. Further, our data show that often the person who overdosed was not the person who had initially been prescribed naloxone, demonstrating that current Swedish legislation concerning THN programs does not reflect the needs of this population.
Also, during the implementation of the THN program, there were frequent requests to the NSP for naloxone from the public, police, security guards, social workers and shelter staff, but current Swedish legislation prevents THN programs from meeting this demand. The inability to supply potential overdose responders with THN is thus a barrier for ensuring the availability of naloxone when and where it is needed. Changing current legislations and making naloxone available over-the-counter are strategies that could increase access for potential bystanders [48].
In the training sessions at the Stockholm NSP, participants were always advised to call for an ambulance and to remain with the person who had overdosed. In our data, an ambulance was called to the scene in 46.6% of the overdoses, which is similar to comparable research [18, 22, 24, 38]. Numerous international studies support the idea that PWUD refrain from calling an ambulance due to fear of police involvement [49–52], losing custody of children or risk of eviction [53–55]. This could well explain why participants did not call an ambulance, since PWUD in Sweden are at risk of being arrested when seeking medical attention in overdose situations as both use and possession of illicit drugs are criminalised. This requires further investigation.
We also observed that 45.3% of reported overdoses took place in a public space. Public drug use may lead to riskier injection practices when individuals are rushed to inject in unsafe environments [56]. International studies conclude that supervised drug consumption sites greatly benefit PWUD, including preventing premature mortality [20]. The high level of overdoses in our study indicates that establishing such sites would provide a safer and clean environment for drug use, especially benefiting PWID living under unstable housing conditions.
We found a higher mortality among those who reported overdose reversals compared to those who did not, which may be related to the high-risk characteristics of this population, i.e. being a man or having a high level of previous overdose experience [8, 57]. However, current lack of information on causes of death among Stockholm NSP clients complicates interpretation of mortality data and requires further study.
Evaluating possible effects of COVID-19 on the study, which impacted half-way through, is not within the scope of this analysis. However, previous research conducted in the Stockholm NSP during the first year of COVID-19, showed that naloxone distribution remained at pre-pandemic levels [58, 59].
A major strength of this study is the large study sample along with linking data to participants’ unique personal identity number, providing opportunity to follow individual participants prospectively.
There are also several limitations. Questionnaires were based on self-reported data which carries the risk for recall- or social desirability bias. The passive follow-up study design relying on spontaneous requests for a refill by THN participants, might lead to misclassification of participants, which may result in underestimation of the number of participants who report naloxone being used, also noted by Siegler et al. [60]. However, our results are strengthened by the many reports of overdose reversals and a large number of refills within a reasonable follow-up time (65.0% within four weeks and 84.7% within three months).
Restricting drug use classification to a primary drug prevented recording information on poly drug use. This limits the conclusions regarding the association between drug use and reports of overdose reversals. This limitation was partly mediated by the study’s method of relating the refill report to the participant’s most recent response on primary drug, capturing changes to an individual’s primary drug use over time.
In accordance with Swedish legislation, clients who enrol in the NSP must verify their identity, as anonymity is not allowed, which is a potential barrier to participation [47]. As a consequence, the reach of the THN program within the larger community of people who are at risk of opioid-overdose is not known, further complicated by the lack of reliable data on overall numbers of PWID in Stockholm. Lastly, since our study population only represent NSP clients, our results may not fully reflect PWID outside an NSP setting.
Conclusions
This study adds to the scarce data on THN programs in Sweden and concludes that the existing framework of an NSP can be utilised to effectively implement a THN program, provide basic training and reach a large number of high-risk individuals. During the four-year study period, THN participants confidently reversed a sizeable number of potentially fatal overdoses, many of which were a result of access to naloxone among participants not primarily using opioids. The rate of naloxone refills was high, indicating that participants were motivated to maintain a supply of naloxone in case of future overdose events.
Current THN programs in Sweden should be expanded to ensure that naloxone is available whenever needed by modifying the restrictions on who can be prescribed or access naloxone. THN programs also need to be supported by a broader and consistent approach to harm reduction where policymakers could consider new interventions such as safer drug consumption sites in order to reduce the risk of overdose fatality.
Supplementary Information
Additional file 1: Table S1a. Adjusted multinomial logistic regression showing naloxone dose endpoints: Dose lost compared to used for overdose reversal. Table S1b. Adjusted multinomial logistic regression showing naloxone dose endpoints: Dose given away compared to used for overdose reversal. Table S1c. Adjusted multinomial logistic regression showing naloxone dose endpoints: Dose stolen compared to used for overdose reversal.
Acknowledgements
The authors wish to acknowledge the staff members and clients in the Stockholm Needle and Syringe Program for making this study possible.
Abbreviations
- CI
Confidence interval
- CPR
Cardio pulmonary resuscitation
- EMCDDA
European Monitoring Centre for Drugs and Drug Addiction
- HCV
Hepatitis C virus
- HIV
Human immunodeficiency virus
- HR
Hazard ratio
- I.m
Intramuscular
- Inf Care NSP
Inf Care needle syringe program
- IRR
Incidence risk ratio
- NSP
Needle and syringe program
- OAT
Opioid substitution therapy
- PWID
People who inject drugs
- PWUD
People who use drugs
- RR
Risk Ratio
- RRR
Relative risk ratio
- THN
Take-Home Naloxone
- U.S
United States
- WHO
World Health Organisation
Authors’ contributions
EH and MK both conceived the study, collected data, drafted the paper, interpreted the results, wrote and edited the paper. AW contributed with data management, statistical analysis, interpretation of results and writing the statistical section. The author(s) read and approved the final manuscript.
Funding
Open access funding provided by Karolinska Institute. This study was funded by grants from Stockholm County Council and the Swedish Research Council for Health, Working life and Welfare. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Availability of data and materials
The datasets used by the study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was performed in accordance with the Helsinki declaration and was approved by The Regional Ethical Review Board in Stockholm (Dnr: 2013/495–31/3, 2015/1374–32 and 2020–03325). Ethical approval included usage of research data from the quality register InfCare NSP. No additional informed consent was needed from participants.
Consent for publication
Not applicable.
Competing interests
MK has received honoraria for lectures/consultancy from AbbVie, Gilead, MSD, Mundipharma, DnE Pharma and Nordic Drugs and has received research grants from Gilead and Nordic Drugs.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Elin Holmén, Email: elin.holmen.2@ki.se.
Anna Warnqvist, Email: anna.warnqvist@ki.se.
Martin Kåberg, Email: martin.kaberg@ki.se.
References
- 1.Ahmad F, Cisewski J, Rossen L, Sutton P. Provisional Drug Overdose Death Counts National Center for Health Statistics. 2022. [Google Scholar]
- 2.Beletsky L, Davis CS. Today’s fentanyl crisis: Prohibition’s Iron Law, revisited. Int J Drug Policy. 2017;46:156–159. doi: 10.1016/j.drugpo.2017.05.050. [DOI] [PubMed] [Google Scholar]
- 3.The European Monitoring Centre for Drugs and Drug Addiction . European Drug Report 2020 Trends and Developments. Luxembourg: Publications Office of the European Union; 2020. [Google Scholar]
- 4.The National Board of Health and Welfare, (Socialstyrelsen) Deaths due to íntoxications from prescribed and illegal substances (Dödsfall till följd av läkemedels- och narkotikaförgiftningar) 2022. [Google Scholar]
- 5.Colledge S, Peacock A, Leung J, Larney S, Grebely J, Hickman M, et al. The prevalence of non-fatal overdose among people who inject drugs: A multi-stage systematic review and meta-analysis. Int J Drug Policy. 2019;73:172–184. doi: 10.1016/j.drugpo.2019.07.030. [DOI] [PubMed] [Google Scholar]
- 6.Thylstrup B, Seid AK, Tjagvad C, Hesse M. Incidence and predictors of drug overdoses among a cohort of >10,000 patients treated for substance use disorder. Drug Alcohol Depend. 2020;206:107714. doi: 10.1016/j.drugalcdep.2019.107714. [DOI] [PubMed] [Google Scholar]
- 7.Warner-Smith M, Darke S, Day C. Morbidity associated with non-fatal heroin overdose. Addiction. 2002;97(8):963–967. doi: 10.1046/j.1360-0443.2002.00132.x. [DOI] [PubMed] [Google Scholar]
- 8.Caudarella A, Dong H, Milloy MJ, Kerr T, Wood E, Hayashi K. Non-fatal overdose as a risk factor for subsequent fatal overdose among people who inject drugs. Drug Alcohol Depend. 2016;162:51–55. doi: 10.1016/j.drugalcdep.2016.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stoové MA, Dietze PM, Jolley D. Overdose deaths following previous non-fatal heroin overdose: record linkage of ambulance attendance and death registry data. Drug Alcohol Rev. 2009;28(4):347–352. doi: 10.1111/j.1465-3362.2009.00057.x. [DOI] [PubMed] [Google Scholar]
- 10.Neale J, Strang J. Naloxone–does over-antagonism matter? Evidence of iatrogenic harm after emergency treatment of heroin/opioid overdose. Addiction. 2015;110(10):1644–1652. doi: 10.1111/add.13027. [DOI] [PubMed] [Google Scholar]
- 11.Martins SS, Sampson L, Cerdá M, Galea S. Worldwide Prevalence and Trends in Unintentional Drug Overdose: A Systematic Review of the Literature. Am J Public Health. 2015;105(11):2373. doi: 10.2105/AJPH.2015.302843a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Håkansson A, Vedin A, Wallin C, Kral AH. Distribution of naloxone to prevent death from heroin overdose. Study of opioid dependent patients’ attitudes to be part of the antidote program. Lakartidningen. 2013;110(29-31):1340–2. [PubMed] [Google Scholar]
- 13.Darke S, Duflou J. The toxicology of heroin-related death: estimating survival times. Addiction. 2016;111(9):1607–1613. doi: 10.1111/add.13429. [DOI] [PubMed] [Google Scholar]
- 14.McDonald R, Campbell ND, Strang J. Twenty years of take-home naloxone for the prevention of overdose deaths from heroin and other opioids-Conception and maturation. Drug Alcohol Depend. 2017;178:176–187. doi: 10.1016/j.drugalcdep.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Behar E, Santos GM, Wheeler E, Rowe C, Coffin PO. Brief overdose education is sufficient for naloxone distribution to opioid users. Drug Alcohol Depend. 2015;148:209–212. doi: 10.1016/j.drugalcdep.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 16.Green TC, Heimer R, Grau LE. Distinguishing signs of opioid overdose and indication for naloxone: an evaluation of six overdose training and naloxone distribution programs in the United States. Addiction. 2008;103(6):979–989. doi: 10.1111/j.1360-0443.2008.02182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones JD, Roux P, Stancliff S, Matthews W, Comer SD. Brief overdose education can significantly increase accurate recognition of opioid overdose among heroin users. Int J Drug Policy. 2014;25(1):166–170. doi: 10.1016/j.drugpo.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clark AK, Wilder CM, Winstanley EL. A systematic review of community opioid overdose prevention and naloxone distribution programs. J Addict Med. 2014;8(3):153–163. doi: 10.1097/ADM.0000000000000034. [DOI] [PubMed] [Google Scholar]
- 19.Sordo L, Barrio G, Bravo MJ, Indave BI, Degenhardt L, Wiessing L, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550. doi: 10.1136/bmj.j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kennedy MC, Karamouzian M, Kerr T. Public Health and Public Order Outcomes Associated with Supervised Drug Consumption Facilities: a Systematic Review. Curr HIV/AIDS Rep. 2017;14(5):161–183. doi: 10.1007/s11904-017-0363-y. [DOI] [PubMed] [Google Scholar]
- 21.Coffin PO, Sullivan SD. Cost-effectiveness of distributing naloxone to heroin users for lay overdose reversal. Ann Intern Med. 2013;158(1):1–9. doi: 10.7326/0003-4819-158-1-201301010-00003. [DOI] [PubMed] [Google Scholar]
- 22.Rowe C, Santos GM, Vittinghoff E, Wheeler E, Davidson P, Coffin PO. Predictors of participant engagement and naloxone utilization in a community-based naloxone distribution program. Addiction. 2015;110(8):1301–1310. doi: 10.1111/add.12961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katzman JG, Greenberg NH, Takeda MY, Moya BM. Characteristics of Patients With Opioid Use Disorder Associated With Performing Overdose Reversals in the Community: An Opioid Treatment Program Analysis. J Addict Med. 2019;13(2):131–138. doi: 10.1097/ADM.0000000000000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Troberg K, Isendahl P, Blomé MA, Dahlman D, Håkansson A. Characteristics of and Experience Among People Who Use Take-Home Naloxone in Skåne County. Sweden Front Public Health. 2022;10:811001. doi: 10.3389/fpubh.2022.811001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eide D, Lobmaier P, Clausen T. Who is using take-home naloxone? An examination of supersavers. Harm Reduct J. 2022;19(1):65. doi: 10.1186/s12954-022-00647-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Troberg K, Isendahl P, Blome MA, Dahlman D, Hakansson A. Protocol for a multi-site study of the effects of overdose prevention education with naloxone distribution program in Skane County, Sweden. BMC Psychiatry. 2020;20(1):49. doi: 10.1186/s12888-020-2470-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The Swedish National Board of Health and Welfare, (Socialstyrelsen) Misuse substance related diagnoses and gambling (Missbruk, substansrelaterade diagnoser och spel om pengar) 2021. [Google Scholar]
- 28.Norwegian Directorate at Health . National Overdose Strategy 2014–2017 Oslo, Norway. 2014. [Google Scholar]
- 29.The National Board of Health and Welfare (Socialstyrelsen). Making naloxone available for patients and individuals outside of healthcare. Opportunities within the current legislation (Tillgängliggöra naloxon för patienter och personer utanför hälso- och sjukvården – Möjligheter inom ramen för dagens rättsliga regleringar). 2017. Available from: https://www.socialstyrelsen.se/globalassets/sharepoint-dokument/artikelkatalog/ovrigt/2017-6-6.pdf.
- 30.The National Board of Health and Welfare (Socialstyrelsen) Information material on Naloxone (Informationsmaterial om naloxon) 2019. [Google Scholar]
- 31.Strang J, McDonald R, Hedrich D, Simon R. Preventing opioid overdose deaths with take-home naloxone. Luxembourg: Publications Office of the European Union; 2016. [Google Scholar]
- 32.Kåberg M, Karlsson N, Discacciati A, Widgren K, Weiland O, Ekström AM, et al. Significant decrease in injection risk behaviours among participants in a needle exchange programme. Infect Dis (Lond) 2020;52(5):336–346. doi: 10.1080/23744235.2020.1727002. [DOI] [PubMed] [Google Scholar]
- 33.Madah-Amiri D. Opioid overdoses and overdose prevention: The establishment of take-home naloxone in Norway. 2017. [Google Scholar]
- 34.Ericson ØB, Eide D, Lobmaier P, Clausen T. Risks and overdose responses: Participant characteristics from the first seven years of a national take-home naloxone program. Drug Alcohol Depend. 2022;240:109645. doi: 10.1016/j.drugalcdep.2022.109645. [DOI] [PubMed] [Google Scholar]
- 35.Bennett AS, Bell A, Doe-Simkins M, Elliott L, Pouget E, Davis C. From Peers to Lay Bystanders: Findings from a Decade of Naloxone Distribution in Pittsburgh. PA J Psychoactive Drugs. 2018;50(3):240–246. doi: 10.1080/02791072.2018.1430409. [DOI] [PubMed] [Google Scholar]
- 36.Enteen L, Bauer J, McLean R, Wheeler E, Huriaux E, Kral AH, et al. Overdose prevention and naloxone prescription for opioid users in San Francisco. J Urban Health. 2010;87(6):931–941. doi: 10.1007/s11524-010-9495-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doe-Simkins M, Quinn E, Xuan Z, Sorensen-Alawad A, Hackman H, Ozonoff A, et al. Overdose rescues by trained and untrained participants and change in opioid use among substance-using participants in overdose education and naloxone distribution programs: a retrospective cohort study. BMC Public Health. 2014;14:297. doi: 10.1186/1471-2458-14-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bennett AS, Bell A, Tomedi L, Hulsey EG, Kral AH. Characteristics of an overdose prevention, response, and naloxone distribution program in Pittsburgh and Allegheny County. Pennsylvania J Urban Health. 2011;88(6):1020–1030. doi: 10.1007/s11524-011-9600-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Banjo O, Tzemis D, Al-Qutub D, Amlani A, Kesselring S, Buxton JA. A quantitative and qualitative evaluation of the British Columbia Take Home Naloxone program. CMAJ Open. 2014;2(3):E153–E161. doi: 10.9778/cmajo.20140008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McAuley A, Munro A, Bird SM, Hutchinson SJ, Goldberg DJ, Taylor A. Engagement in a National Naloxone Programme among people who inject drugs. Drug Alcohol Depend. 2016;162:236–240. doi: 10.1016/j.drugalcdep.2016.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buresh M, Gicquelais RE, Astemborski J, Kirk GD, Mehta SH, Genberg BL. Fatal overdose prevention and experience with naloxone: A cross-sectional study from a community-based cohort of people who inject drugs in Baltimore, Maryland. PLoS ONE. 2020;15(3):e0230127. doi: 10.1371/journal.pone.0230127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McDonald R, Parkin S, Eide D, Neale J, Clausen T, Metrebian N, et al. Rethinking ‘carriage’ of take-home naloxone. Int J Drug Policy. 2021;95:103253. doi: 10.1016/j.drugpo.2021.103253. [DOI] [PubMed] [Google Scholar]
- 43.Kenney SR, Anderson BJ, Bailey GL, Stein MD. Factors associated with naloxone administration in an opioid dependent sample. J Subst Abuse Treat. 2018;84:17–20. doi: 10.1016/j.jsat.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dayton L, Gicquelais RE, Tobin K, Davey-Rothwell M, Falade-Nwulia O, Kong X, et al. More than just availability: Who has access and who administers take-home naloxone in Baltimore, MD. PLoS ONE. 2019;14(11):e0224686. doi: 10.1371/journal.pone.0224686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McAuley A, Matheson C, Robertson JR. From the clinic to the street: the changing role of benzodiazepines in the Scottish overdose epidemic. Int J Drug Policy. 2022;100:103512. doi: 10.1016/j.drugpo.2021.103512. [DOI] [PubMed] [Google Scholar]
- 46.Young S, Williams S, Otterstatter M, Lee J, Buxton J. Lessons learned from ramping up a Canadian Take Home Naloxone programme during a public health emergency: a mixed-methods study. BMJ Open. 2019;9(10):e030046. doi: 10.1136/bmjopen-2019-030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Värmå Falk M, Strömdahl S, Ekström AM, Kåberg M, Karlsson N, Dahlborn H, et al. A qualitative study of facilitators and barriers to participate in a needle exchange program for women who inject drugs. Harm Reduct J. 2020;17(1):84. doi: 10.1186/s12954-020-00425-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davis C, Webb D, Burris S. Changing law from barrier to facilitator of opioid overdose prevention. J Law Med Ethics. 2013;41(Suppl 1):33–36. doi: 10.1111/jlme.12035. [DOI] [PubMed] [Google Scholar]
- 49.Wagner KD, Valente TW, Casanova M, Partovi SM, Mendenhall BM, Hundley JH, et al. Evaluation of an overdose prevention and response training programme for injection drug users in the Skid Row area of Los Angeles. CA Int J Drug Policy. 2010;21(3):186–193. doi: 10.1016/j.drugpo.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baca CT, Grant KJ. What heroin users tell us about overdose. J Addict Dis. 2007;26(4):63–68. doi: 10.1300/J069v26n04_08. [DOI] [PubMed] [Google Scholar]
- 51.Latimore AD, Bergstein RS. "Caught with a body" yet protected by law? Calling 911 for opioid overdose in the context of the Good Samaritan Law. Int J Drug Policy. 2017;50:82–89. doi: 10.1016/j.drugpo.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 52.Koester S, Mueller SR, Raville L, Langegger S, Binswanger IA. Why are some people who have received overdose education and naloxone reticent to call Emergency Medical Services in the event of overdose? Int J Drug Policy. 2017;48:115–124. doi: 10.1016/j.drugpo.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karamouzian M, Kuo M, Crabtree A, Buxton JA. Correlates of seeking emergency medical help in the event of an overdose in British Columbia, Canada: Findings from the Take Home Naloxone program. Int J Drug Policy. 2019;71:157–163. doi: 10.1016/j.drugpo.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 54.Ambrose G, Amlani A, Buxton JA. Predictors of seeking emergency medical help during overdose events in a provincial naloxone distribution programme: a retrospective analysis. BMJ Open. 2016;6(6):e011224. doi: 10.1136/bmjopen-2016-011224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lankenau SE, Wagner KD, Silva K, Kecojevic A, Iverson E, McNeely M, et al. Injection drug users trained by overdose prevention programs: responses to witnessed overdoses. J Community Health. 2013;38(1):133–141. doi: 10.1007/s10900-012-9591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trayner KMA, McAuley A, Palmateer NE, Goldberg DJ, Shepherd SJ, Gunson RN, et al. Increased risk of HIV and other drug-related harms associated with injecting in public places: national bio-behavioural survey of people who inject drugs. Int J Drug Policy. 2020;77:102663. doi: 10.1016/j.drugpo.2020.102663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mathers BM, Degenhardt L, Bucello C, Lemon J, Wiessing L, Hickman M. Mortality among people who inject drugs: a systematic review and meta-analysis. Bull World Health Organ. 2013;91(2):102–123. doi: 10.2471/BLT.12.108282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McDonald R, Eide D, Abel-Ollo K, Barnsdale L, Carter B, Clausen T, et al. A rapid assessment of take-home naloxone provision during COVID-19 in Europe. Int J Drug Policy. 2022;107:103787. doi: 10.1016/j.drugpo.2022.103787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lindqvist K, Wallmofeldt C, Holmén E, Hammarberg A, Kåberg M. Health literacy and changes in pattern of drug use among participants at the Stockholm Needle Exchange Program during the COVID-19 pandemic. Harm Reduct J. 2021;18(1):52. doi: 10.1186/s12954-021-00499-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Siegler A, Huxley-Reicher Z, Maldjian L, Jordan R, Oliver C, Jakubowski A, et al. Naloxone use among overdose prevention trainees in New York City: A longitudinal cohort study. Drug Alcohol Depend. 2017;179:124–130. doi: 10.1016/j.drugalcdep.2017.06.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1a. Adjusted multinomial logistic regression showing naloxone dose endpoints: Dose lost compared to used for overdose reversal. Table S1b. Adjusted multinomial logistic regression showing naloxone dose endpoints: Dose given away compared to used for overdose reversal. Table S1c. Adjusted multinomial logistic regression showing naloxone dose endpoints: Dose stolen compared to used for overdose reversal.
Data Availability Statement
The datasets used by the study are available from the corresponding author on reasonable request.


