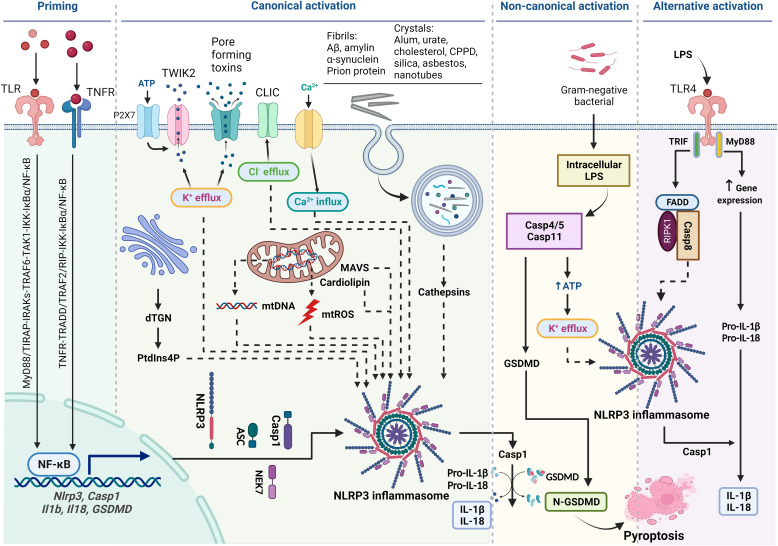
Fig. 1.
Diagram of activation of NLPR3 inflammasome. Priming is induced by signal 1, such as LPS and TNF-α, that upregulates the transcription of genes encoding NLRP3 inflammasome components by activating their membrane-bound pattern recognition receptors. Canonical activation of NLRP3 inflammasome is elicited by signal 2 including PAMPs, such as nigericin, viral RNA, and MDP, and DAMPs, such as extracellular ATP, mtDNA, and mtROS, and particles and crystals. Activation involves multiple signaling events including K+ efflux, Ca2+ flux, Cl–efflux, lysosomal disruption, mtROS production, and release of oxidized mtDNA. Activation may also involve docking of NLRP3 at mitochondrial outer membrane via mitochondrial antiviral signaling protein or cardiolipin and at dispersed dTGN through PtdIns4P. Formation of NLRP3 inflammasome includes binding of NLRP3 with NEK7, oligomerization of NLRP3, assembly of ASCs into fibrils, and recruitment and activation of Casp1. Casp1 in turn activates Pro-IL-1β and Pro-IL-18 and GSDMD by proteolytic cleavage of the proteins. IL-1β and IL-18 are secreted to promote proinflammatory responses, whereas N-GSDMD forms pores in the plasma membrane to result in pyroptosis of the cell. Noncanonical activation of NLRP3 inflammasome is induced by gram-negative bacteria. Release of LPS from engulfed bacteria into the cytoplasm activates human Casp4/5 or mouse Casp11, which cleaves GSDMD to induce pyroptosis and indirectly activates the NLRP3 inflammasome to activate Casp1 and IL-1β and IL-18. Noncanonical activation does not require priming as Casp4 is present at a high level. The alternative pathway of activation is elicited by TLR4 agonists like LPS, which activates the TLR4-TRIF-RIPK1-FADD-Casp8 signaling. Casp8 activates the NLRP3 inflammasome but does not require K+ efflux, ASC speck formation, or pyroptosis.