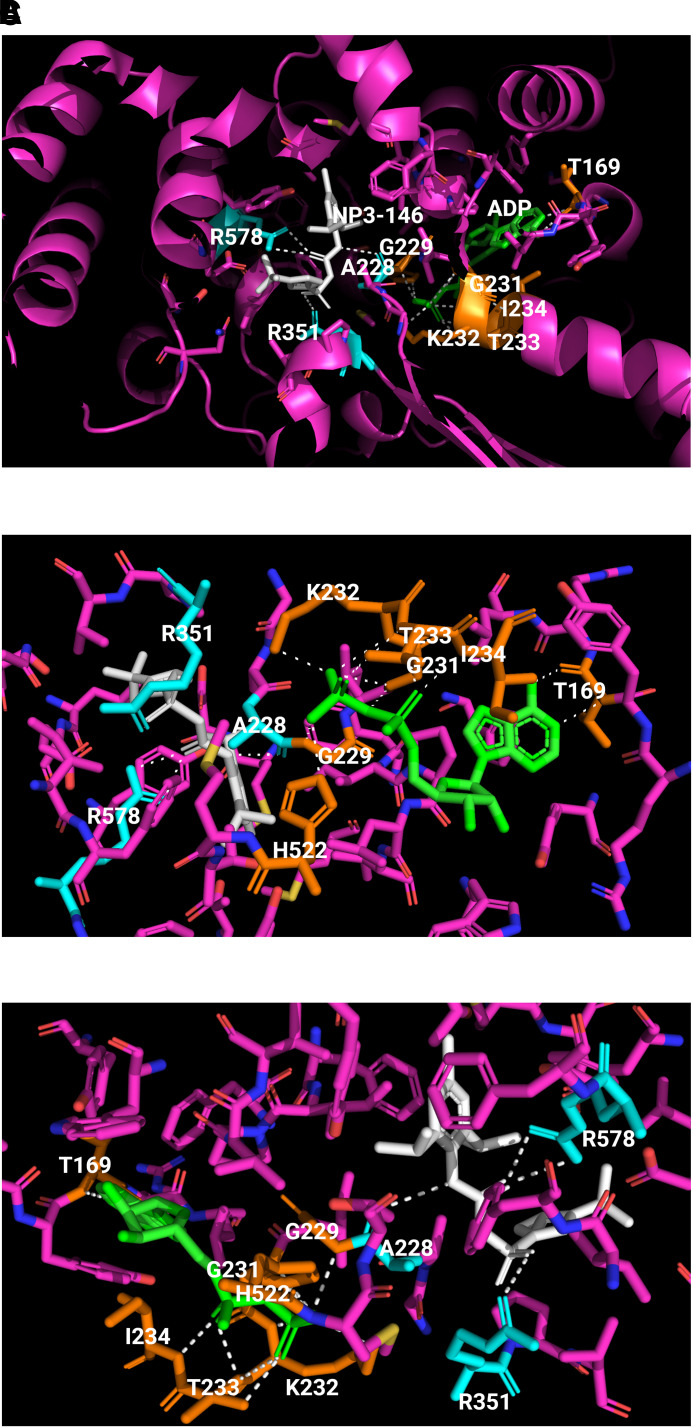
Fig. 4.
Binding of ADP and an MCC950 analog inhibitor in inactive NLRP3. The crystal structure of human NACHT bound with ADP and MCC950 analog NP3-146 is used to illustrate the binding sites and formation of polar bonds with residues for ADP and NP3-146. In the structure, bound ADP is shown in green, bound NP3-146 in white, residues within 5 Å of a ligand in yellow for the ADP binding site or in cyan for the NP3-146 binding site, and all other residues in magenta. Polarized bonds are shown with dotted line in white. (A) Crystal structure of human NLRP3 NACHT bound with ADP and NP3-146 (PDB: 7ALV) (Dekker et al., 2021). NLRP3 NACHT displays an overall configuration characteristic of NACHT STAND proteins. ADP and NP3-146 bind in adjacent but separate binding pockets. (B) The ADP binding site from A. The β-phosphate of ADP forms a polar contact with His522 from the WHD subdomain. ADP makes extensive interactions with the Walker A motif (226-GAAGIGKT-233) including polar bonds with Gly229, Gly231, Lys232, Thr233, and Ile234. Its adenine group forms polar bonds with Thr169. (C) The NP3-146 binding site from A. NP3-146 binds to the opposite side of Walker A from ADP binding. Its urea group forms a crucial polar contact with Ala228 of Walker A. The inhibitor also forms important interactions with Arg578 of HD2, whereas its sulfonyl group interacts with Arg351 of NBD.