Abstract
Introduction:
Preterm children with bronchopulmonary dysplasia (BPD) frequently require supplemental oxygen in the outpatient setting. In this study, we sought to determine patient characteristics and demographics associated with need for supplemental oxygen at initial hospital discharge, timing to supplemental oxygen liberation, and associations between level of supplemental oxygen and likelihood of respiratory symptoms and acute care usage in the outpatient setting.
Methods:
A retrospective analysis of subjects with BPD on supplemental oxygen (O2) was performed. Subjects were recruited from outpatient clinics at Johns Hopkins University and the Children’s Hospital of Philadelphia between 2008 and 2021. Data were obtained by chart review and caregiver questionnaires.
Results:
Children with BPD receiving ≥ 1 liter of O2 were more likely to have severe BPD, pulmonary hypertension and be older at initial hospital discharge. Children discharged on higher levels of supplemental O2 were slower to wean to room air compared to lower O2 groups (p<0.001). Additionally, weaning off supplemental O2 in the outpatient setting was delayed in children with gastrostomy tubes and those prescribed inhaled corticosteroids, on public insurance or with lower household incomes. Level of supplemental O2 at discharge did not influence outpatient acute care usage or respiratory symptoms.
Conclusion:
BPD severity and level of supplemental oxygen use at discharge did not correlate with subsequent acute care usage or respiratory symptoms in children with BPD. Weaning of O2 however was significantly associated with socioeconomic status and respiratory medication use, contributing to the variability in O2 weaning in the outpatient setting.
Keywords: Bronchopulmonary dysplasia, supplemental oxygen, Socioeconomic status, outpatient
Introduction
Preterm birth comprises 10.1% of all births in the United States.1 With advances in neonatal care, survival rates of premature infants have improved, especially for those born at extremely low birth weights.2 These low birth weight infants are at highest risk for developing bronchopulmonary dysplasia (BPD) and to minimize hypoxemia events, are more likely to be discharged to home on supplemental oxygen. In a single center study, approximately 1/3 of preterm infants with BPD who were followed in the outpatient setting were initially discharged to home on oxygen therapy.3 Clinical practice guidelines from both the American Thoracic Society and British Thoracic Society recommend home oxygen therapy for infants with BPD with ongoing hypoxemia.4 5 However, variations in supplemental oxygen use and weaning strategies in children with BPD,6 may influence outpatient respiratory outcomes, particularly during the first three years of life.
BPD is characterized by alveolar hypoplasia and small airway disease. Achieving normal oxygen levels in children with BPD can help promote somatic growth, optimize developmental outcomes and minimize the development or worsening of pulmonary hypertension.7 In the outpatient setting however, few guidelines for weaning supplemental oxygen in children with BPD exist; thus variability in respiratory outcomes and timing to liberation from supplemental oxygen is common. When comparing children with BPD on supplemental oxygen at discharge to those on room air several studies have reported higher rates of hospitalizations and healthcare utilization in those discharged on supplemental oxygen.8,9 However, another study found no differences in these outcomes between the two groups.10
In this study, we focused on children with BPD discharged on supplemental oxygen (O2). The purpose of this study was to determine whether an association existed between level of supplemental oxygen use at discharge and subsequent outpatient acute care usage and respiratory symptoms in this study population. Secondly, we sought to identify patient characteristics and demographics that may be associated with the variability in the timing of liberation from supplemental oxygen in children with BPD, following initial hospital discharge.
Methods
A retrospective chart review was performed on subjects (n=417) recruited from outpatient BPD clinics at the Children’s Hospital of Philadelphia and Johns Hopkins Children’s Center between 2008–2021. All subjects were born at less than 37 weeks-gestation and were diagnosed with BPD by NIH consensus definition,11 and were on home supplemental oxygen via nasal cannula; patients with tracheostomies or home ventilators were excluded. This study was approved by Johns Hopkins Institutional Review Board (Protocol #NA_00051884) and the Children’s Hospital of Pediatrics Institutional Review Board (IRB# 20–017614) and all caregivers were consented at time of presentation to clinic.
Children using supplemental oxygen at initial hospital discharge were divided into four groups. Those who required ≤1/8 lpm of oxygen (group 1), those who required >1/8 lpm of oxygen but <1/2 lpm of oxygen (group 2), those who required ≥1/2 lpm but <1 lpm of oxygen (group 3), and those who required ≥1 lpm of oxygen (group 4). Demographic data and clinical characteristics were collected by chart review. Birthweight percentile was corrected by gestational age.12 Race/ethnicity was obtained through caregiver report. Median household income was estimated using residential zip codes based on U.S. Census data.
Acute care usage, defined as emergency department visits and hospitalizations, and chronic respiratory symptom data was obtained via caregiver-completed questionnaires at outpatient visits when subjects were between 0 and 3 years of age.
Statistics:
Chi square and ANOVA tests were performed to compare demographic data and clinical characteristics between oxygen groups (Table 1). Cox regressions adjusted for inter-site variation were performed to identify factors associated with earlier or later liberation from supplemental oxygen (Table 2). Associations between amount of oxygen at initial hospital discharge and acute care use/chronic respiratory symptoms were assessed for via logistic regression models adjusted for inter-site variation, potential demographic/clinical confounders, duration of oxygen use, number of questionnaires, and age of questionnaire assessments (Table 3). Results that were statistically significant had a P value less than or equal to 0.05. Stata IC 16 was used for data analysis.
Table 1:
Demographic and Clinical Characteristics by Oxygen Amount at Initial Discharge
| Mean ± S.D. [Range] | Entire Study Population (n=417) | ≤0.125 LPM (n=149) | >0.125 and <0.5 LPM (n=130) | ≥0.5 and <1.0 LPM (n=111) | ≥1.0 LPM (n=27) | P value | |
|---|---|---|---|---|---|---|---|
| Sex (% male) | 55.6% | 55.7% | 53.9% | 63.1% | 33.3% | 0.044 | |
| Race (% non-white; n=404) | 59.7% | 59.5% | 66.4% | 54.3% | 50.0% | 0.20 | |
| Ethnicity (% Hispanic; n=415) | 5.5% | 5.4% | 4.6% | 4.6% | 14.8% | 0.18 | |
| Gestational age (weeks) | 26.4 ± 2.6 [22.9, 36.9] | 26.3 ± 2.4 [22.9, 36.9] | 26.5 ± 2.7 [23.0, 35.5] | 26.2 ± 2.4 [23.0, 35.3] | 26.8 ± 2.6 [23.4, 36.0] | 0.69 | |
| Birthweight (grams; n=409) | 858 ± 408 [380, 3370] | 868 ± 399 [380, 3370] | 875 ± 455 [390, 3181] | 829 ± 302 [415, 1790] | 836 ± 590 [420, 2790] | 0.82 | |
| Birthweight percentile (%; n=409) | 40 ± 26 [1, 95] | 42 ± 27 [1, 95] | 39 ± 24 [1, 89] | 42 ± 26 [1, 89] | 25 ± 19 [2, 67] | 0.018 | |
| Small for gestational age (% < 10th percentile; n=409) | 13.7% | 13.2% | 13.1% | 12.7% | 24.0% | 0.49 | |
| BPD severity (%; n=387) | Mild | 0.5% | 1.4% | 0.0% | 0.0% | 0.0% | <0.001 |
| Moderate | 34.4% | 47.5% | 38.8% | 17.8% | 7.7% | ||
| Severe | 65.1% | 51.1% | 61.2% | 82.2% | 92.3% | ||
| Gastrostomy tube (% yes) | 33.1% | 16.8% | 33.1% | 46.9% | 66.7% | <0.001 | |
| Nissen fundoplication in subjects with gastrostomies (% yes; n =138) | 65.2% | 52.0% | 60.5% | 71.2% | 77.8% | 0.22 | |
| Pulmonary hypertension after 36 weeks (% yes) | 27.8% | 16.1% | 24.6% | 40.5% | 55.6% | <0.001 | |
| Pulmonary hypertension medications in subjects with pulmonary hypertension (% yes; n=116) | 40.5% | 16.7% | 25.0% | 53.3% | 73.3% | <0.001 | |
| Diuretics (% yes) | 65.5% | 54.4% | 70.0% | 73.0% | 74.1% | 0.005 | |
| Inhaled corticosteroids (% yes) | 74.8% | 65.8% | 77.7% | 79.3% | 92.6% | 0.005 | |
| Initial discharge age (months; n=416) | 4.7 ± 2.5 [0.1, 17.9] | 4.0 ± 2.1 [0.5, 15.3] | 4.7 ± 2.3 [0.1, 14.9] | 5.3 ± 2.8 [1.0, 17.9] | 5.8 ± 3.2 [0.2, 13.6] | <0.001 | |
| Initial discharge age (weeks post-menstrual age; n=416) | 46.7 ± 10.5 [34.7, 105.1] | 43.6 ± 9.1 [34.7, 98.4] | 46.9 ± 9.6 [35.4, 93.0] | 49.4 ± 11.6 [36.3, 105.1] | 52.0 ± 12.6 [35.9, 87.0] | <0.001 | |
| Median household income ($ in thousands; n=413) | 77.2 ± 27.9 [20.4, 156.8] | 81.1 ± 27.8 [28.5, 156.8] | 72.4 ± 26.7 [22.8, 156.8] | 76.1 ± 28.6 [20.4, 145.8] | 83.5 ± 28.9 [31.9, 148.9] | 0.042 | |
| Public insurance (% yes) | 53.2% | 48.3% | 53.1% | 55.9% | 70.4% | 0.18 | |
Table 2:
Likelihood of weaning oxygen in a given month by demographic data clinical characteristics
| Hazard Ratio (Univariate Model)* | P value | Hazard Ratio (Multivariate Model)** | P value | ||
|---|---|---|---|---|---|
| Amount of oxygen at initial discharge (LPM) | 0.26 | <0.001 | 0.41 | <0.001 | |
| Sex (male=0; female=1) | 0.95 | 0.64 | - | - | |
| Race (white=0; nonwhite=1) | 1.21 | 0.09 | - | - | |
| Ethnicity (non-Hispanic=0; Hispanic=1) | 0.94 | 0.80 | - | - | |
| Gestational age (months) | 1.01 | 0.52 | - | - | |
| Birthweight (grams) | 1.00 | 0.99 | - | - | |
| Birthweight percentile (%) | 1.00 | 0.31 | - | - | |
| Small for gestational age (no=0; yes=1) | 0.95 | 0.76 | - | - | |
| BPD severity (Reference=Mild) | Moderate | 1.84 | 0.56 | - | - |
| Severe | 1.21 | 0.85 | - | - | |
| Gastrostomy tube (no=0; yes=1) | 0.38 | <0.001 | 0.57 | <0.001 | |
| Nissen fundoplication (no=0; yes=1) | 0.39 | <0.001 | - | - | |
| Pulmonary hypertension after 36 weeks (no=0; yes=1) | 0.57 | <0.001 | - | - | |
| Pulmonary anti-hypertensive medications (no=0; yes=1) | 0.45 | <0.001 | - | - | |
| Diuretics (no=0; yes=1) | 0.81 | 0.06 | - | - | |
| Inhaled corticosteroids (no=0; yes=1) | 0.31 | <0.001 | 0.39 | <0.001 | |
| Discharge age (months) | 0.85 | <0.001 | 0.94 | 0.016 | |
| Median household income ($, in thousands) | 1.00 | 0.019 | 1.01 | 0.001 | |
| Public insurance (no=0; yes=1) | 0.78 | 0.023 | - | - | |
Hazard ratios were generated through Cox regression and adjusted for inter-site variation with a dummy variable.
Model was generated via backwards stepwise regression using variables significant in univariate modeling also with adjustment for site; final model n=412.
Table 3:
Acute care usage between oxygen groups
| Adjusted odds ratio for outcome by initial oxygen amount (LPM)* | P value | |
|---|---|---|
| Emergency department visit (no=0; yes=1) | 0.42 [0.11, 1.62] (n = 229) |
0.21 |
| Hospital admission (no=0; yes=1) | 0.95 [0.25, 3.64] (n = 230) |
0.94 |
| Systemic steroid use (no=0; yes=1) | 1.53 [0.40, 5.76] (n = 230) |
0.53 |
| Antibiotic use (no=0; yes=1) | 0.76 [0.21, 2.77] (n = 230) |
0.68 |
| Cough and/or wheeze (no=0; yes=1) | 0.60 [0.18, 2.04] (n = 230) |
0.41 |
| Rescue medication use (no=0; yes=1) | 0.59 [0.17, 2.00] (n = 228) |
0.40 |
| Shortness of breath with activity (no=0; yes=1) | 0.64 [0.16, 2.61] (n = 208) |
0.53 |
| Nighttime symptoms (no=0; yes=1) | 2.45 [0.62, 9.60] (n = 229) |
0.20 |
Odds ratios were generated through logistic regression and adjusted for duration of oxygen use, mean age for assessments of outcomes, center site, number of times outcomes were assessed, sex, birthweight percentile, severity of BPD (dummy variable), gastrostomy tube, pulmonary hypertension after 36 weeks, use of pulmonary hypertension medications, diuretic use, inhaled steroid use, and median household income.
Results
Subject Demographics
At initial hospital discharge, children who required higher levels of supplemental oxygen were more likely to have severe BPD, lower birthweight percentiles, and a diagnosis of pulmonary hypertension compared to those who required less supplemental oxygen (Table 1). There were no differences in birthweight or percent who were small for gestational age. Additionally, higher levels of supplemental oxygen at initial hospital discharge were associated with older age at discharge (months and weeks post-menstrual age both p<0.001), gastrostomy tube placement (p<0.001), and use of diuretics (p=0.005), inhaled corticosteroids (ICS) (p=0.005), and pulmonary anti-hypertension medications (p<0.001). Race/ethnicity and public insurance were not associated with higher supplemental oxygen use at initial hospital discharge; however, there was some variation in oxygen amounts by median household income (p=0.042).
Timing of weaning of supplemental oxygen in BPD children in the outpatient setting
Liberation from supplemental oxygen was delayed in children who were initially discharged on higher amounts of supplemental oxygen (p<0.001) (Figure 1). The median age at liberation was 8.4 months for group 1, 10.8 months for group 2, 13.5 months for group 3, and 19.6 months for group 4.
Figure 1.
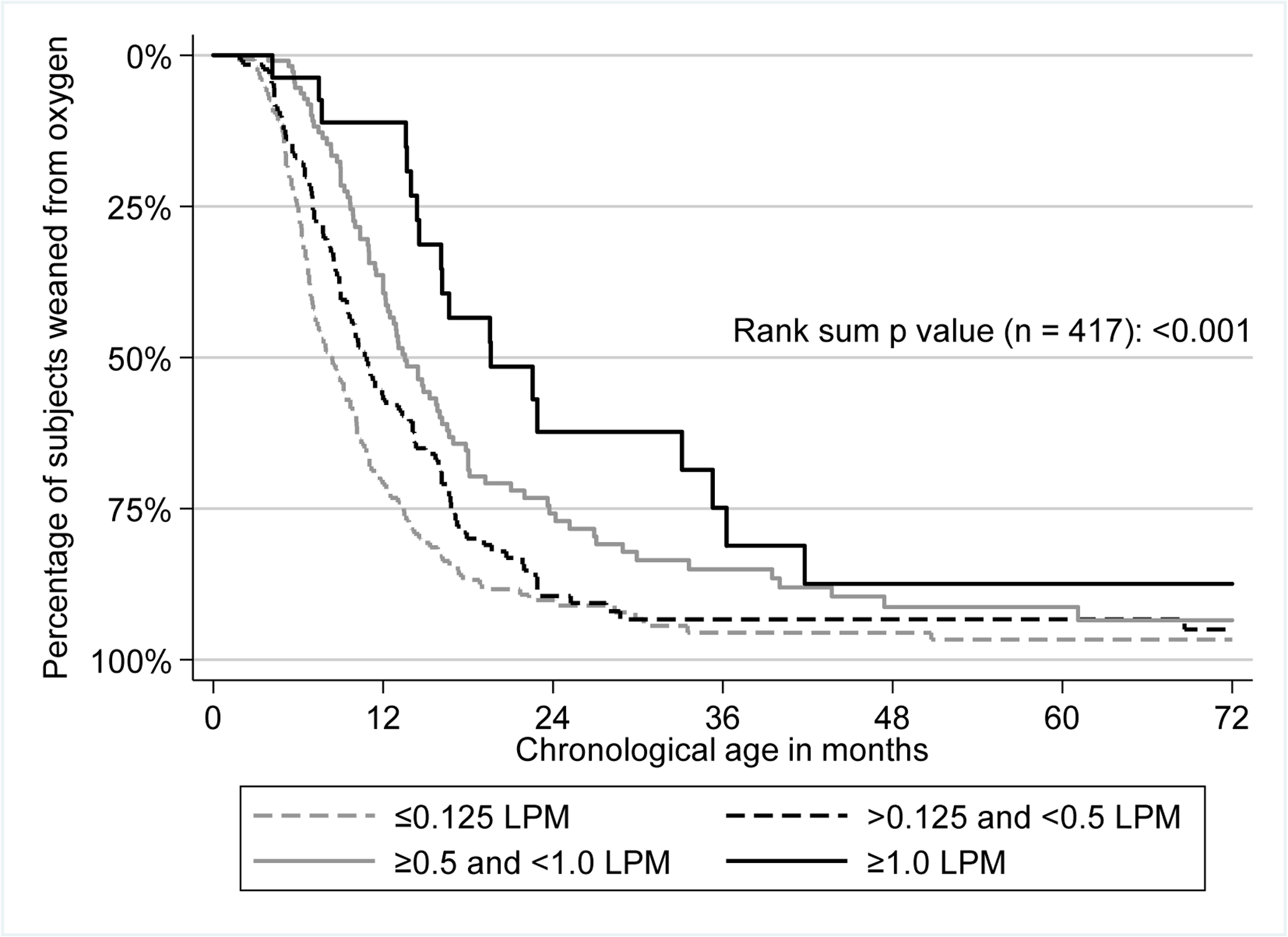
Kaplan-Meier plot with time to weaning from home supplemental oxygen by amount of oxygen at initial hospital discharge
Likelihood of weaning supplemental oxygen in a given month, in the outpatient setting
In a multivariate model, the likelihood of being weaned off supplemental oxygen in a given month was lower if the child was prescribed more supplemental oxygen initially, had a gastrostomy tube, had a lower estimated household income, or was prescribed inhaled corticosteroids (Table 2). Interestingly, severity of BPD at 36 weeks corrected age did not influence the likelihood of weaning off supplemental oxygen in a given month.
Acute care usage and respiratory outcomes, with regard to level of supplemental oxygen at initial hospital discharge.
We next examined correlations between outpatient respiratory outcomes and acute care usage with the level of supplemental oxygen use at initial hospital discharge. When examining differences by amount of oxygen at initial discharge, we found no significant differences in acute care usage, chronic respiratory symptoms, or rescue medication use (Table 3). Raw outcome data can be found in Supplemental Table 1.
Discussion
The burden of respiratory morbidities in children with BPD can be lifelong13 and identifying those at highest risk for long-term respiratory morbidities, is challenging. Children with BPD who require supplemental oxygen at initial hospital discharge have varying degrees of cardiopulmonary involvement and are likely at higher risk for chronic respiratory symptoms during the pre-school years when compared to children discharged off supplemental oxygen.14 Identifying factors that predict outpatient acute care usage, chronic respiratory symptoms and timing to oxygen liberation could help modify respiratory morbidities in these children. This study focused on children with BPD discharged to home on supplemental oxygen to address these issues. Not unexpectantly, children discharged on higher levels of supplemental oxygen were more likely to have severe BPD and to carry the diagnosis of pulmonary hypertension. Additionally, those who required higher levels of supplemental oxygen at initial hospital discharge were more likely to have lower birthweight percentiles and to be older at initial hospital discharge. However, children discharged on higher levels of supplemental oxygen did not have a higher likelihood of acute care usage, chronic respiratory symptoms or need for respiratory medications during acute illnesses when compared to those discharged on lower levels of supplemental oxygen. The likelihood of weaning supplemental oxygen, in a given month, was significantly lower in children with gastrostomy tubes, children prescribed inhaled corticosteroids (ICS) and in those who lived in homes with lower estimated incomes. Findings from this study suggest that although severity of BPD influences level of supplemental oxygen at initial hospital discharge, other factors after hospital discharge influence weaning of supplemental oxygen and respiratory morbidities, including socioeconomic status and ICS use, which could be modifiable factors.
In this study, several risk factors were associated with delayed weaning of supplemental oxygen. In particular, we found that weaning oxygen, per given month was less likely in children with lower estimated household incomes. This finding suggest that socioeconomic status can be a factor in liberating a child from supplemental oxygen in the outpatient setting. This finding raises the question of whether children with lower SES, have more difficulties in accessing care, once they are in the outpatient setting. However, a recent single center study did not support this.15 There are other factors however, related to socioeconomic status, that likely influence pulmonary health outcomes in patients with BPD. These may include indoor and outdoor air pollution, proximity to major highways and neighborhood poverty.16 17 18Additional studies will be needed to determine if other health disparities or perceptions due to SES, influence variations in oxygen weaning strategies in children with BPD.
We also found that higher use of ICS was associated with delayed weaning of supplemental oxygen. It is possible that ICS was used as an additive therapy in those who were more difficult to wean from supplemental oxygen, which may account for delayed weaning of supplemental oxygen. Other reasons may also affect weaning in the outpatient setting. Wong et. al., studied infants with moderate or severe BPD discharged on varying amounts of supplemental oxygen. They found that shorter NICU stays were associated with quicker oxygen weans at 9 and 12 months, with no correlation to birthweight or gestational age.19 Our findings indicate that factors outside of the NICU can influence weaning of supplemental oxygen in children with BPD.
In this study, no differences in acute care usage or respiratory symptoms were found between any of the oxygen groups in children with BPD. Higher levels of supplemental oxygen at discharge were not associated with increased rates of emergency room visits, hospitalizations, systemic steroid use, or antibiotic use for respiratory conditions. There were also no differences in chronic respiratory symptoms or rescue medication use between the four oxygen groups. It is possible that supplemental oxygen use in the outpatient setting, regardless of level administered, can attenuate respiratory symptom severity. Greenough et. al., reported that children with BPD between the ages of 2 to 4 years, who required supplemental oxygen did not have increased hospital admissions, compared to those on room air.9 However, their study did see an increase in wheezing and use of inhalers. Lodha et. al.,10 also examined respiratory outcomes at 3 years of age in children without BPD, with BPD, and with BPD on supplemental oxygen. Similar to Greenough’s study they found that children with BPD on supplemental oxygen did not have higher rates of hospitalization or antibiotic use compared to the other groups. Unlike our study however, Lodha et. al., did not stratify by amount of supplemental oxygen use. Other studies however have shown higher rates of rehospitalization for respiratory issues in children with BPD who require oxygen supplementation at home.8,19
In our study the level of oxygen support was relatively narrow between groups. Nevertheless, children prescribed higher levels of supplemental oxygen at discharge were liberated from supplemental oxygen later than those prescribed lower levels of supplemental oxygen at discharge. Although children prescribed a higher level of supplemental oxygen at initial hospital were more likely to have severe BPD, severity of BPD did not correlate with outpatient emergency room visits, hospitalizations, systemic steroid use, or antibiotic use for respiratory conditions. Jensen et. al., found that mode of respiratory support, regardless of oxygen therapy at 36 weeks PMA was more predictive of death or respiratory morbidity at 18 to 26 months corrected age,20 therefore BPD classification based on oxygen requirement at 36 weeks PMA, as used in this study, may not be optimal for predicting respiratory outcomes post discharge.11 Alternatively, although not quantified in our study, immunizations for influenza and RSV may have attenuated differences in primary outcomes among children receiving different levels of supplemental oxygen support at discharge.
A limitation of this study is the retrospective nature of the study design. Furthermore, this study included patients from two centers, in which the demographics of these cohorts predominately represent an urban population, which may not be generalizable to other patient populations, particularly those in rural areas. Both centers in this study also have outpatient BPD clinics which may account for higher comfort in discharging children with BPD on higher levels of oxygen in the outpatient setting. Nevertheless, our study results suggest that the use of supplemental oxygen can help to mitigate differences in BPD severity with regard to acute care usage and reported respiratory symptoms in children with BPD in the outpatient setting.
Another limitation of the study is that we did not collect data on the total number of patients discharged on supplemental oxygen, given that patients presented from various NICUs across both regions. Additionally, while the amount of supplemental oxygen at discharge was collected and we assume that these are four distinct oxygen groups, weight at body discharge was not collected so that we cannot definitively confirm patient overlaps.21
In summary, among BPD children on supplemental oxygen in the outpatient setting, the level of oxygen supplementation at initial hospital discharge was not shown to correlate with acute care usage or respiratory symptoms. Weaning of supplemental O2 however was significantly associated with household income and ICS use, indicating that these factors can influence timing of oxygen weaning by healthcare providers in the outpatient setting.
Supplementary Material
Funding/Support:
This work was supported by the National Institutes of Health (Bethesda, MD, USA)(SAM: R01 HL114800), the Johns Hopkins Eudowood Foundation (BCA: Fellowship grant), and the Children’s Hospital of Philadelphia (JMC).The funding sources had no involvement in the writing of the manuscript or the decision to submit.
References
- 1.Martin JA, Hamilton BE & Osterman MJK Births in the United States, 2019. NCHS Data Brief, 1–8 (2020). [PubMed] [Google Scholar]
- 2.Bell EF et al. Mortality, In-Hospital Morbidity, Care Practices, and 2-Year Outcomes for Extremely Preterm Infants in the US, 2013–2018. Jama 327, 248–263, doi: 10.1001/jama.2021.23580 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeh J, McGrath-Morrow SA & Collaco JM Oxygen weaning after hospital discharge in children with bronchopulmonary dysplasia. Pediatr Pulmonol 51, 1206–1211, doi: 10.1002/ppul.23442 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayes D Jr. et al. Home Oxygen Therapy for Children. An Official American Thoracic Society Clinical Practice Guideline. American journal of respiratory and critical care medicine 199, e5–e23, doi: 10.1164/rccm.201812-2276ST (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balfour-Lynn IM et al. BTS guidelines for home oxygen in children. Thorax 64 Suppl 2, ii1–26, doi: 10.1136/thx.2009.116020 (2009). [DOI] [PubMed] [Google Scholar]
- 6.Lagatta J, Clark R & Spitzer A Clinical predictors and institutional variation in home oxygen use in preterm infants. The Journal of pediatrics 160, 232–238, doi: 10.1016/j.jpeds.2011.08.033 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Everitt LH et al. Weaning oxygen in infants with bronchopulmonary dysplasia. Paediatric respiratory reviews 39, 82–89, doi: 10.1016/j.prrv.2020.10.005 (2021). [DOI] [PubMed] [Google Scholar]
- 8.DeMauro SB et al. Home Oxygen and 2-Year Outcomes of Preterm Infants With Bronchopulmonary Dysplasia. Pediatrics 143, doi: 10.1542/peds.2018-2956 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenough A et al. Preschool healthcare utilisation related to home oxygen status. Arch. Dis. Child Fetal Neonatal Ed 91, F337–F341 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lodha A et al. Does chronic oxygen dependency in preterm infants with bronchopulmonary dysplasia at NICU discharge predict respiratory outcomes at 3 years of age? Journal of perinatology : official journal of the California Perinatal Association 35, 530–536, doi: 10.1038/jp.2015.7 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Jobe AH & Bancalari E Bronchopulmonary dysplasia. Am J Respir Crit Care Med 163, 1723–1729, doi: 10.1164/ajrccm.163.7.2011060 (2001). [DOI] [PubMed] [Google Scholar]
- 12.Gonçalves J et al. Cannabis and Its Secondary Metabolites: Their Use as Therapeutic Drugs, Toxicological Aspects, and Analytical Determination. Medicines (Basel) 6, doi: 10.3390/medicines6010031 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collaco JM & McGrath-Morrow SA Bronchopulmonary dysplasia as a determinant of respiratory outcomes in adult life. Pediatr Pulmonol, doi: 10.1002/ppul.25301 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin H et al. Home oxygen use and 1-year outcome among preterm infants with bronchopulmonary dysplasia discharged from a Chinese regional NICU. Front Pediatr 10, 978743, doi: 10.3389/fped.2022.978743 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aoyama BC, McGrath-Morrow SA & Collaco JM Socioeconomic status and outpatient follow-up in children with bronchopulmonary dysplasia. Pediatr Pulmonol, doi: 10.1002/ppul.26232 (2022). [DOI] [PubMed] [Google Scholar]
- 16.Deschamps J et al. Neighborhood Disadvantage and Early Respiratory Outcomes in Very Preterm Infants with Bronchopulmonary Dysplasia. The Journal of pediatrics 237, 177–182.e171, doi: 10.1016/j.jpeds.2021.06.061 (2021). [DOI] [PubMed] [Google Scholar]
- 17.Banwell E et al. Area Deprivation and Respiratory Morbidities in Children with Bronchopulmonary Dysplasia. Pediatr Pulmonol, doi: 10.1002/ppul.25969 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collaco JM, Morrow M, Rice JL & McGrath-Morrow SA Impact of road proximity on infants and children with bronchopulmonary dysplasia. Pediatr Pulmonol 55, 369–375, doi: 10.1002/ppul.24594 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong MD, Neylan M, Williams G, Zahir SF & Chawla J Predictors of home oxygen duration in chronic neonatal lung disease. Pediatr Pulmonol 56, 992–999, doi: 10.1002/ppul.25257 (2021). [DOI] [PubMed] [Google Scholar]
- 20.Jensen EA et al. The Diagnosis of Bronchopulmonary Dysplasia in Very Preterm Infants. An Evidence-based Approach. American journal of respiratory and critical care medicine 200, 751–759, doi: 10.1164/rccm.201812-2348OC (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benaron DA & Benitz WE Maximizing the stability of oxygen delivered via nasal cannula. Arch Pediatr Adolesc Med 148, 294–300, doi: 10.1001/archpedi.1994.02170030064015 (1994). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


