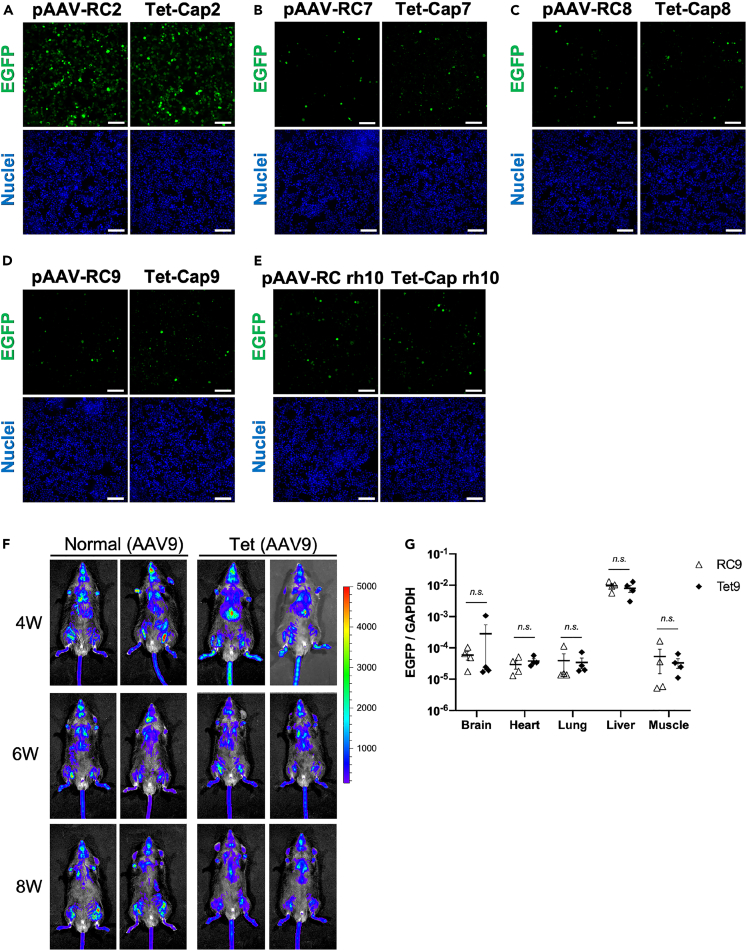
Figure 5.
Novel AAV vector production system does not affect viral infectivity in vitro and in vivo
(A–E) (A–D) Infectivity data of adeno-associated virus (AAV) vectors for various serotypes in vitro. The 2v6.11 cells were infected with AAV2 (500 vg/cell), AAV7 (1000 vg/cell), AAV8 (1000 vg/cell), AAV9 (1000 vg/cell), and AAVrh10 (1000 vg/cell). Cells were observed at 96 h after infection. Data indicate the infectivity of the AAV vector (EGFP; green) in AAV2 (A), AAV7 (B), AAV8 (C), AAV9 (D), and AAVrh10 (E). The blue signal shows nuclei (Hoechst 33342). White bar shows 100 μm.
(F) Data for AAV vector distribution in vivo. C57BL/6 mice were injected with AAV9 vectors carrying an EGFP-P2A-Nluc gene (5 × 1010 vg/mouse) derived from normal control (RC9) and Tet-Cap9 systems through the retro-orbital vein. Luciferase signals were monitored using IVIS-CT after injection of luciferase substrate through the retro-orbital vein at 4, 6, and 8 weeks. Data shows representative mice of RC9 (n = 4) and Tet-Cap9 (n = 4). Bar colors correspond to luciferase signals with a range of 0–5000 photons/s.
(G) AAV vector genome detection in indicated organs at 3 weeks after injection. Data were normalized with the AAV vector (EGFP) and mouse genomic GAPDH values. White triangles and black rhombuses indicate data of each mouse for RC9 (n = 4) and Tet-Cap9 (n = 4), respectively. Letters in the panel indicate the following: n.s. = not significant. Error bars indicate the standard error of the mean.