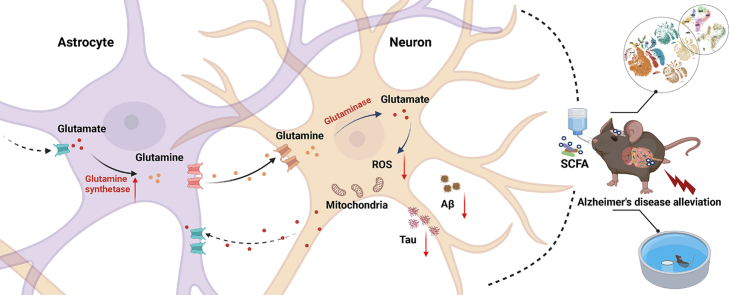
Abstract
The brain is particularly susceptible to oxidative damage which is a key feature of several neurodegenerative diseases, including Alzheimer's disease (AD), Parkinson's disease (PD) and Huntington's disease. The shuttling of glutathione (GSH) precursors from astrocytes to neurons has been shown to be instrumental for the neuroprotective activity. Here, we revealed that short chain fatty acids (SCFA), which have been related to AD and PD, could promote glutamate-glutamine shuttle to potentially resist oxidative damage in neurons at cellular level. Furthermore, we performed nine-month-long dietary SCFA supplementations in APPswe/PS1dE9 (APP/PS1) mice, and showed that it reshaped the homeostasis of microbiota and alleviated the cognitive impairment by reducing Aβ deposition and tau hyperphosphorylation. Single-cell RNA sequencing analysis of the hippocampus revealed SCFA can enhance astrocyte-neuron communication including glutamate-glutamine shuttle, mainly by acting on astrocyte in vivo. Collectively, our findings indicate that long-term dietary SCFA supplementations at early aging stage can regulate the neuroenergetics to alleviate AD, providing a promising direction for the development of new AD drug.
Keywords: Alzheimer's disease, Short chain fatty acids, Astrocyte, ROS, Neuroenergetics, Glutamate-glutamine shuttle
Graphical abstract
Highlights
-
•
SCFA contribute to the glutamate-glutamine shuttle by acting on glutamine synthetase.
-
•
Long-term SCFA diet alleviates cognitive and spatial memory defeat in APP/PS1 mice.
-
•
Long-term SCFA diet reduces Aβ deposition and abnormal phosphorylation of tau.
-
•
SCFA promote astrocyte-neuron metabolic coupling to reduce oxidative damage.
1. Introduction
Central neural system (CNS) diseases, especially the Alzheimer's disease (AD), are major health burdens in aging society [1]. AD is an archetypical neurodegenerative and metabolic disease characterized by the amyloid β-protein plaques (Aβ), neurofibrillary tangles, synapse damage, disorders of cerebral energy metabolism, mitochondrial dysfunction, and oxidative stress [[2], [3], [4]]. Remarkably, these pathological manifestations have occurred decades before the cognitive deficits observed clinically, and the patients in the early stage are asymptomatic [5,6]. Traditional intervention strategies directly targeting the pathology have resulted in high failure rate of clinical trials, and it is urgent to track disease progression and promote drug development in a non-traditional way [7].
An imbalance between reactive oxygen species production and antioxidant defences is a typical trigger of neuronal damage and death, as has been demonstrated in AD patients with associated biomarkers of oxidative damage to DNA, lipids and proteins, particularly in areas rich in neuritic plaques [[8], [9], [10], [11]]. Oxidative damage is a crucial contributor to the impairment of ATP production and brain energy metabolism, and sirtuins disorders induce oxidative damage to mitochondrial DNA, leading to defects in energy production and neuronal damage in AD [12]. Sophisticated glucose metabolism processes are essential to dysfunction in mild cognitive impairment and AD, and a wide range of metabolic intermediates such as lactate, glutamate and pyruvate assist in brain energy production.
Along with the aging process, the impairment of brain energy metabolism contributes to neurological dysfunction and constitutes a vicious circle, especially in AD [13,14]. Neuronal energy hypometabolism such as excessive fatty acids, insufficient energy substrates and impaired metabolism-related enzyme function are direct evidences. Astrocytes, the most numerous cell type in the brain, are the mainstay of metabolic homeostasis and protection for neurons. As reported, astrocytes provide metabolic substrates to neurons and assist in their antioxidation by recycling neurotransmitter glutamate, delivering lactate, and endocytosing neuron-derived lipid particles [[15], [16], [17]]. Admittedly, neuronal self-rescue patterns show a high dependence on astrocyte replenishment to resist oxidative damage [18].
The SCFA (mainly acetate, propionate, and butyrate) produced by bacterial fermentation of dietary fiber have been recognized as the pivotal molecules regulating neurodegenerative disease including AD, Huntington's disease, and Parkinson's disease (PD) [19,20]. Butyrate as a histone deacetylase inhibitor could improve associative learning memory in aged APP/PS1 mice without affecting Aβ deposition [21], while it will reduce plaques and inhibit glial cell activation and inflammatory responses in adult 5xFAD mice [22,23]. It is estimated that SCFA are responsible for 6–10% of the body's daily energy need and the brain consumes up to 20% [16,24]. Similarly, SCFA act as a substrate reservoir for glucose and lipids to support the host energy requirements [25,26]. In colonocytes, butyrate alone can cover up to 70% of the energy source and germ-free mice show impaired mitochondrial respiration [27,28]. In brain, SCFA reshape mitochondrial lipid metabolism in vivo and in vitro leading to neuronal death, notably in phosphatidylethanolamine, mitochondrial lipid carnitine and cardiolipid metabolism [29]. Conversely, butyrate protects brain regions from mitochondrial function and behavioural changes [30]. Propionate, as a substrate of intestinal gluconeogenesis, can activate gluconeogenesis, and the signal can improve host metabolism through the intestinal-brain neural circuit [31]. However, little is known about the metabolic interaction of SCFA on astrocytes and neurons during the aging process.
Here, we found that SCFA rescue glutamate delivery deficits in astrocyte-neuron coupling by regulating glutamine synthetase (GS). The long-term SCFA dietary in APP/PS1 mice can increase the SCFA producer in the gut microbiota, and then alleviate the cognition defects. Further scRNA sequencing analysis of the mice hippocampus revealed SCFA can enhance astrocyte-neuron communication mainly by acting on astrocyte and promote glutamate-glutamine shuttle to protect neurons against oxidative damage. Together, these data indicate that long-term dietary SCFA supplementations in early aging can positively influence the brain energy metabolism, with important implications for the development of new AD drug.
2. Results
2.1. SCFA mitigate neuronal oxidative damage by acting on glutamine synthetase
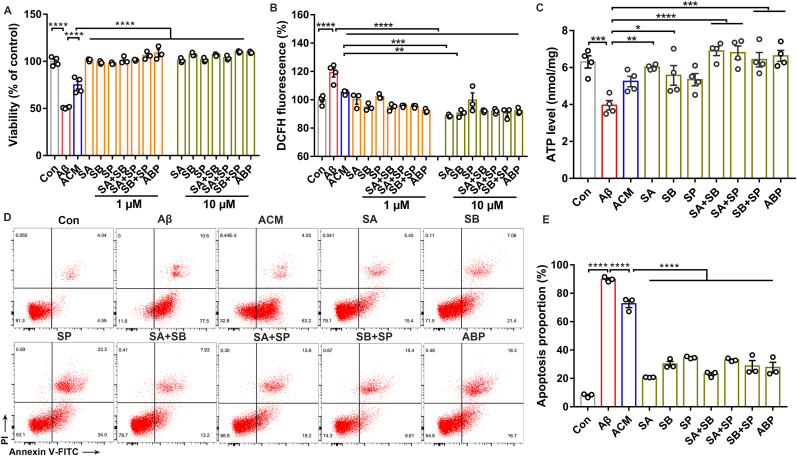
To investigate whether astrocyte-associated signaling regulates neuronal activity in SCFA intervention, we performed in vitro incubation of neurons (PC12 cell) after Aβ injury with astrocyte-conditioned medium (ACM), which was formed by stimulating astrocytes (C6 cell) with different combinations of SCFA. We found that SCFA-contained ACM significantly increased neuronal viability compared to astrocytes alone, especially with mixture of sodium acetate (SA), sodium butyrate (SB) and sodium propionate (SP) (Fig. 1A). We further hypothesized that the secretions produced by SCFA-treated astrocytes may exert a protective effect on neurons. The DCFH fluorescent probe test showed a significant increase of neuronal ROS levels after Aβ injury. Strikingly, the ROS level can be reduced by SCFA-contained ACM (Fig. 1B). We then observed that SCFA significantly alleviated the Aβ-induced decreases in ATP levels and reduced the proportion of apoptotic cells (Fig. 1C–E). Our results show that SCFA counteract neuronal oxidative stress and mitochondrial damage to exert neuroprotection by stimulating astrocytes.
Fig. 1.
SCFA alleviate neuronal oxidative stress injury and exert neuroprotection. (A) The effect of astrocyte-conditioned medium (ACM) formed by incubating astrocytes (C6 cells) with or without different concentrations of SCFA (1 μM, 10 μM) on Aβ-induced neurons (PC12 cells) for 24 h using CCK8. (SA: sodium acetate; SB: sodium butyrate; SP: sodium propionate; ABP: SA + SB + SP) (n = 3–4 per group; ****p < 0.0001; one-way ANOVA). (B) ROS levels in Aβ-induced neurons (PC12 cells) exposed to SCFA-ACM (1 μM, 10 μM) for 24 h (n = 3–4 per group; ****p < 0.0001, ***p = 0.0002, **p = 0.0011; one-way ANOVA). (C) ATP levels in Aβ-induced neurons (PC12 cells) by SCFA-ACM (10 μM) for 24 h (n = 4 per group; Con vs Aβ: ***p = 0.0008; Aβ vs SA/SB: **p = 0.0040, *p = 0.0436; Aβ vs SA + SB/SA + SP: ****p < 0.0001; Aβ vs SB + SP/ABP: ***p = 0.0004/0.0001; one-way ANOVA). (D) Apoptosis detection by Annexin V-FITC/PI double staining in Aβ-induced neurons (PC12 cells) by SCFA-ACM (10 μM) for 24 h. (E) Quantitative analysis of (D) (n = 3 per group; ****p < 0.0001; one-way ANOVA).
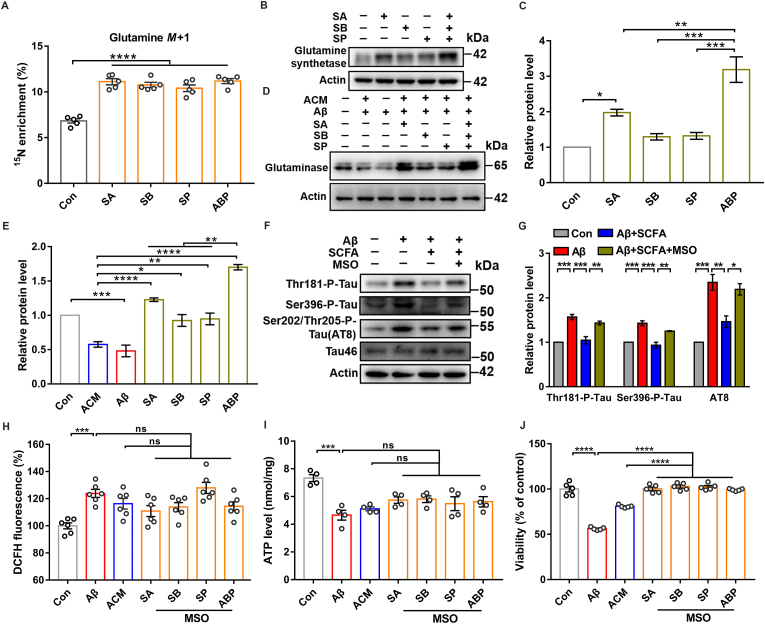
Given that excess glutamate in the synaptic gap is taken up by astrocytes and converted to glutamine as a raw material for glutathione synthesis against oxidative stress in neurons, we evaluated the glutamine level with isotope labeling (15NH4Cl) in astrocytes by supplementing SCFA. SCFA-treated astrocytes had higher levels of glutamine, indicating the enhanced activity of GS (Fig. 2A). Immunoblotting assay further confirmed that SCFA treatment of astrocytes increases the level of the GS protein (Fig. 2B and C), which promotes the uptake of glutamate in the synaptic gap and then converts it to glutamine for glutathione synthesis against oxidative stress in neurons [18]. Subsequently, we explored the downstream effects of SCFA on glutamine shuttled into neurons. Aβ injury caused a significant down-regulation of neuronal glutaminase expression levels, and SCFA-contained ACM was able to restore its abnormal expression (Fig. 2D and E).
Fig. 2.
SCFA regulate glutamine synthetase to promote astrocyte-neuron glutamate-glutamine shuttle. (A) LC-MS assay of the relative level of 15N-labeled glutamine (M+1) produced by astrocytes (C6 cells) after SCFA intervention for 24 h (n = 5 per group; ****p < 0.0001; one-way ANOVA). (B) Western blot detection of glutamine synthetase (GS) levels in astrocytes (C6 cells) after SCFA intervention at 24 h. (C) Quantitative analysis of (B) (n = 3 per group; Con vs SA: *p = 0.0188; SA/SB/SP vs ABP: **p = 0.0046/***p = 0.0001/***p = 0.0002; one-way ANOVA). (D) Western blot detection of glutaminase expression levels in Aβ-induced neurons (PC12 cells) exposed to SCFA-ACM (10 μM) for 24 h. (E) Quantitative analysis of (D) (n = 3 per group; Con vs Aβ: ***p = 0.0004; ACM vs SA/SB/SP: ****p < 0.0001/*p = 0.0148/**p = 0.0092; ABP vs SA/SB/SP: **p = 0.0011/****p < 0.0001/****p < 0.0001; one-way ANOVA). (F) Hippocampal primary neurons and astrocytes were isolated and cultured in vitro using a co-culture system established by Transwell, with astrocytes in the upper chamber and neurons in the lower chamber, and astrocytes were preincubated with SCFA or MSO (2 mM) for 24 h, followed by co-culture with Aβ-induced neurons for 24 h, and neurons were collected for test. Effect of MSO and SCFA on neuronal tau protein phosphorylation levels in a Transwell co-culture system by western blot. (G) Quantitative analysis of (F) (n = 3 per group; Thr181, ***p = 0.0003 (left), ***p = 0.0006 (middle), **p = 0.0043 (right); Ser396, ***p = 0.0006 (left), ***p = 0.0002 (middle), **p = 0.0041 (right); AT8, ***p = 0.0003 (left), **p = 0.0051 (middle), *p = 0.0156 (right); one-way ANOVA). (H) DCFH-staining-based assessment of ROS levels in Aβ-induced neurons (PC12 cells) after exposure to the SCFA-ACM with or without GS inhibitor MSO (2 mM) (n = 6 per group; Con vs Aβ: ***p = 0.0004; ns: not significant; one-way ANOVA).(I) ATP levels in Aβ-induced neurons (PC12 cells) by SCFA-ACM with or without GS inhibitor MSO for 24 h (n = 4 per group; Con vs Aβ: ***p = 0.0001; ns: not significant; mean ± s.e.m; one-way ANOVA). (J) The cell viability of astrocyte-conditioned medium (ACM) formed by incubating astrocytes (C6 cells) with or without SCFA (10 μM) or GS inhibitor MSO (2 mM) on Aβ-induced neurons (PC12 cells) for 24 h using CCK8. (n = 5 per group; ****p < 0.0001; ns: not significant; one-way ANOVA).
All data are presented as the mean ± s.e.m.
We further verified the role of GS in the astrocyte-neuron glutamate-glutamine shuttle using GS inhibitor methionine sulfoximine (MSO). After incubation of astrocytes with MSO and SCFA, the alleviation effect of SCFA-contained ACM on Aβ-induced neuronal oxidative damage was abrogated, as evidenced by the fact that ROS levels remained abnormally elevated (Fig. 2H). We then isolated and cultured primary neurons and astrocytes and established a co-culture system using transwell. Western blot data showed that SCFA treatment significantly reduced neuronal tau protein hyperphosphorylation levels, while MSO administration can abrogate the reduction (Fig. 2F and G). In addition, MSO also eliminated the regulation of ATP levels by SCFA but did not affect cell viability (Fig. 2I and J). Therefore, we conclude that the neuroprotective effect of SCFA is carried out by counteracting oxidative stress via enhancing GS activity, and then increasing glutamine levels in the astrocyte-neuron glutamate-glutamine cycle.
2.2. Dietary SCFA alleviate cognitive deficits
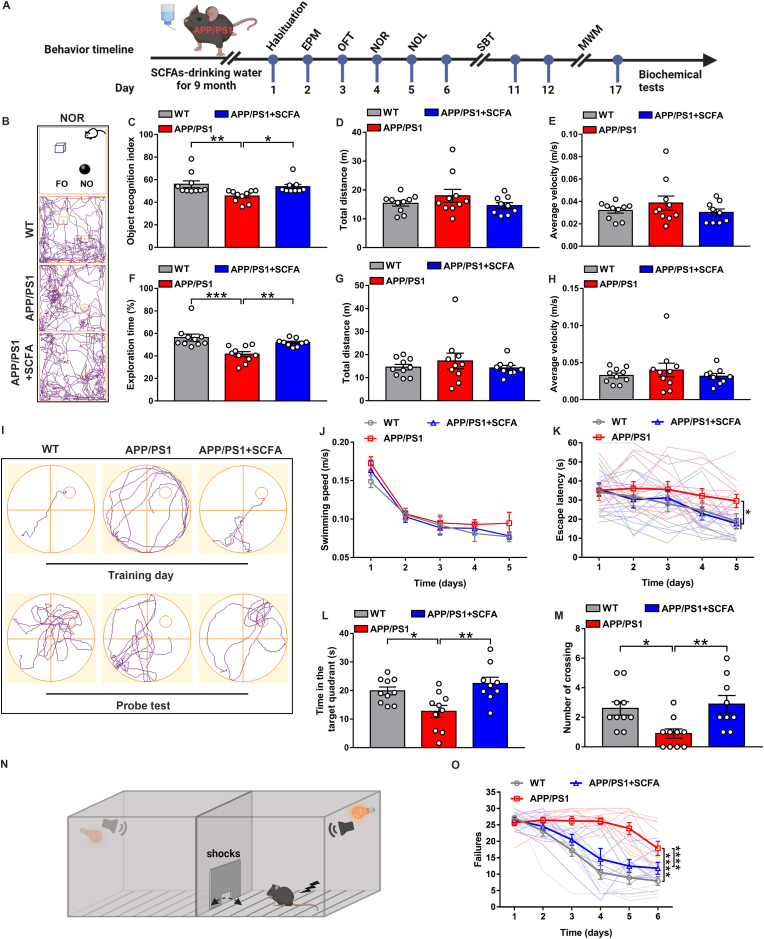
Based on our observations of the energy metabolic regulation by SCFA in vitro, we directly administered dietary SCFA supplementations to 5-month-old APP/PS1 mice for 9 months. Behavioral tests were then performed on 14-month-old APP/PS1 mice (Fig. 3A), including anxiety-like behavior tests, elevated plus maze (EPM) and open field test (OFT), recognition and spatial memory tests, novel object recognition (NOR), novel object location (NOL), two-way shuttle box test (SBT) and Morris water maze (MWM), on each group of mice in turn [32].
Fig. 3.
Long-term dietary SCFA supplementations prevent deficits of recognition and memory in elderly APP/PS1 mice. (A) Schematic diagram for the long-term dietary supplementation with SCFA and behavior tests in APP/PS1 mice (created with BioRender.com). (B–H) Recognition behavioral tests after SCFA treatment for 9 months were performed in 14-month-old APP/PS1 mice. Representative motion trajectories in novel object recognition (NOR) and novel object location (NOL) test. Images show the experimental details and operating procedures, as well as the trajectory tracking of each group of mice in NOR (FO: familiar Object, NO: novel Object) (B). The object recognition index (C) (**p = 0.0068; *p = 0.0383, one-way ANOVA) total distance (D) and average velocity (E) were recorded in NOR. Exploration time (F) (***p = 0.0003; **p = 0.0095, one-way ANOVA) total distance (G) and average velocity (H) were analyzed in NOL. (I–M) The Morris water maze test to assess the effect of the dietary SCFA on spatial memory in 14-month-old APP/PS1 mice. Representative illustrations show the trajectories of maze on the fifth training day and the final probe test of each experimental group (I). On training days, the swimming speed (J) and escape latency (time for mice to find the platform) (K) were evaluated (Normal diet: *p = 0.0347; SCFA diet: *p = 0.0432; two-way ANOVA with Tukey's test). The residence time of the target quadrant where the platform is located (L) (*p = 0.0224, **p = 0.0026; one-way ANOVA) and the number of times the virtual platform crosses (M) (*p = 0.0233, **p = 0.0097; one-way ANOVA) were evaluated in each group of mice in final probe test. (N–O) Dietary SCFA effect on failures in two-way shuttle box test (SBT). A schematic for shuttle box operation is presented (N); the number of failures in active avoidance response for each experimental group during the 6 days of training (O) (****p < 0.0001; two-way ANOVA with Tukey's test).
All data are presented as the mean ± s.e.m. WT, n = 10 mice; normal diet, n = 10 APP/PS1 mice; SCFA diet, n = 9 APP/PS1 mice.
We found in EPM results that the APP/PS1 mice had no significant difference in the number of entries and staying time in closed and open arms compared with the WT mice, and no abnormality was observed after SCFA feedings (Figs. S1A–C), and in OFT no significant trend fluctuations in the number of entries and time spent in the central area, and in the total movement distance of the mice in each group (Fig S1E, G and H). However, we found that SCFA feedings can slightly improve the spontaneous activity and exploratory behavior disorder of APP/PS1 mice, which was manifested by increasing the movement distance of the central area (Figs. S1D and F). The above results indicated that the elderly APP/PS1 mice had no obvious anxiety-like phenotypes, and the dietary SCFA supplementations showed little effect.
In the NOR/NOL tests, the APP/PS1 mice had significantly lower recognition index and novel location exploration time than the WT mice, while the SCFA supplementations group had similar values to WT mice (Fig. 3B, C and F) and was not affected in total distance and average velocity (Fig. 3D, E, G and H). MWM tests spatial memory of mice by finding underwater hidden platform, and as the training time increases, the APP/PS1 mice still spent significantly more time to find the platform, whereas the mice from WT and SCFA supplementations group found the platform faster and had similar memory capabilities (Fig. 3I and K). There was no difference in the swimming speed of the mice in each group (Fig. 3J). In probe test, similar to WT group, the mice from SCFA supplementations group had significantly longer exploration time in the target quadrant where the platform was located, and the number of crossing the virtual platform location was significantly increasing compared to the APP/PS1 group (Fig. 3I, L and M). In SBT, significantly faster avoidance behavior was observed for mice from SCFA supplementations group compared to the APP/PS1 group, and the number of failures in active avoidance response was close to the WT group (Fig. 3N and O). Together, these data suggest dietary SCFA slow down the process of cognitive deficits and improves the object recognition and spatial memory in elderly APP/PS1 mice.
2.3. Dietary SCFA slow down the pathological process
We assessed the effect of dietary SCFA supplementations on gut microbiota of APP/PS1 mice and found that there are different composition characteristics, with a significant increase in the abundance of Akkermansia and Clostridium_XIVb after 2 months of SCFA supplementations, compared to the APP/PS1 mice (Figs. S2A–E). Consistently, LEfse analysis revealed a higher abundance of SCFA-producing bacteria (Anaerofustis) after 9 months of SCFA supplementations (Figs. S2F and G). In addition, we found no obvious effect of the dietary SCFA supplementations on gastrointestinal function in APP/PS1 mice (Fig. S1I-O).
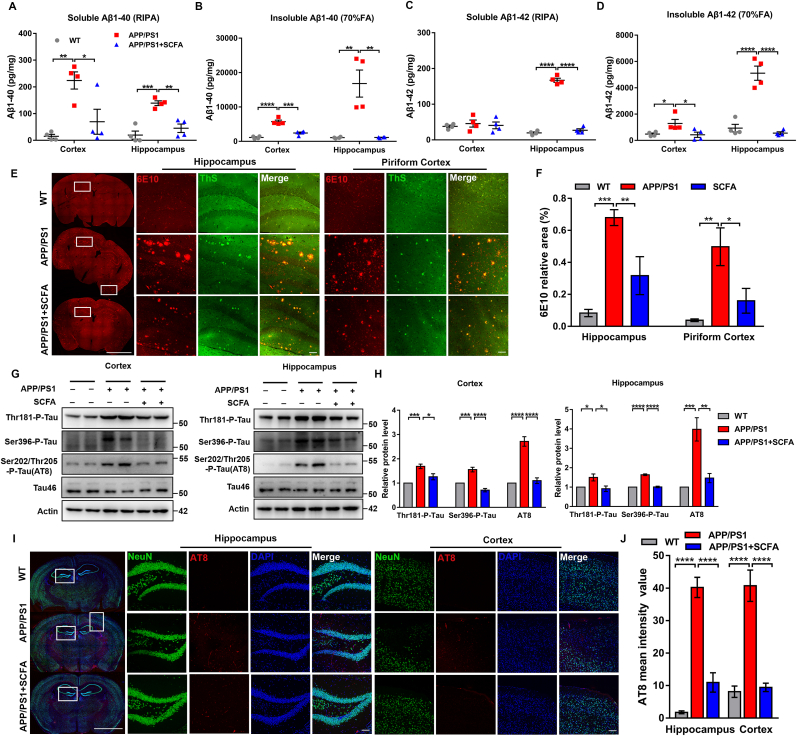
We then investigated whether dietary SCFA supplementations affected to the AD pathological process. ELISA tests confirmed that SCFA supplementations significantly reduced the soluble and insoluble Aβ (1–40/1-42) levels in the cortex and hippocampus of APP/PS1 mice (Fig. 4A–D). Subsequently, we scanned the whole brain slice to examine the Aβ deposition through co-localization immunostaining of 6E10 (multiple forms of Aβ: APP, oligomer and plaque) and ThS (plaque form of Aβ). 6E10 immunoreactivity was detected in the hippocampus and cortex of APP/PS1 mice (piriform and somatosensory cortex), but 6E10-positive areas were significantly reduced after SCFA supplementations (Fig. 4E). Further laser confocal and immunohistochemistry observations also showed that plaques were significantly reduced, and Aβ deposition was significantly alleviated in DG, CA1 and piriform cortex (Fig. 4E and F and S3A-C) of mice from the SCFA supplementations group, compared to the APP/PS1 group.
Fig. 4.
Dietary SCFA reduce amyloid deposition and tau protein hyperphosphorylation in elderly APP/PS1 mice. (A, B) Relative levels of Aβ1-40 in RIPA-cleaved soluble proteins (A) and 70% FA-cleaved insoluble proteins (B) in the hippocampus and cortex in 14-month-old WT, normal diet APP/PS1, and SCFA diet APP/PS1 mice (n = 4 mice; a. Cortex: **p = 0.0029, *p = 0.0166; Hippocampus: ***p = 0.0004, **p = 0.0023. b. Cortex: ****p < 0.0001, ***p = 0.0001; Hippocampus: **p = 0.0016; one-way ANOVA). (C, D) as in (A-B) for soluble (C) and insoluble (D) of Aβ1-42 (n = 4 mice; c. Hippocampus: ****p < 0.0001. d. Cortex: *p = 0.0481 (left), *p = 0.0368 (right); Hippocampus: ****p < 0.0001; one-way ANOVA). (E) Immunofluorescence co-localization with ThS dye (green) and anti-Aβ (6E10, red) antibody on whole brain slices (left, scale bar, 1 mm), the hippocampus (middle, scale bar, 100 μm), and the piriform cortex (right, scale bar, 100 μm) of 14-month-old WT, normal diet APP/PS1, and SCFA diet APP/PS1 mice. (F) Quantitative analysis of (E) (n = 3 mice, bilateral statistics; Hippocampus: ***p = 0.0001, **p = 0.0075; Piriform cortex: **p = 0.0023, *p = 0.0191; one-way ANOVA). (G) Immunoblotting for the phosphorylation levels of the tau protein (targeting Thr181, Ser396, Ser202/Thr205 (AT8) in the hippocampus and cortex in 14-month-old WT, normal diet APP/PS1, and SCFA diet APP/PS1 mice. (H) Quantitative analysis of (g) (n = 4 mice; Cortex: Thr181, ***p = 0.0008, *p = 0.0148; Ser396, ***p = 0.0005, ****p < 0.0001; AT8, ****p < 0.0001. Hippocampus: Thr181, *p = 0.0450 (left), *p = 0.0224 (right); Ser396, ****p < 0.0001; AT8, ***p = 0.0006, **p = 0.0019; one-way ANOVA). (I) Immunofluorescence with anti-NeuN (green) and anti-AT8 (red) antibody on whole brain slices (left, scale bar, 1 mm), hippocampus (middle, scale bar, 100 μm) and cortex (right, scale bar, 100 μm) in 14-month-old WT, normal diet APP/PS1, and SCFA diet APP/PS1 mice. (J) Quantitative analysis of (i) (n = 3 mice, bilateral statistics; ****p < 0.0001; one-way ANOVA).
All data are presented as the mean ± s.e.m. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Moreover, using antibodies targeting Thr181, Ser396, Ser202/Thr205 (AT8), tau phosphorylation was examined in the hippocampus and cortex of APP/PS1 and SCFA supplementations group. The results showed that SCFA supplementations reduced the abnormal increase of tau phosphorylation levels in the cortex and hippocampus of APP/PS1 mice, but without changes in tau46 expression level (Fig. 4G and H). Further immunostaining with NeuN and AT8 antibodies showed that the tau phosphorylation levels in the hippocampus (DG and CA1) and cortex was significantly reduced in the SCFA supplementations group, in comparison to APP/PS1 mice (Fig. 4I and J and S3D). Overall, our data suggest dietary SCFA supplementations can reduce amyloid plaque deposition and abnormal phosphorylation of tau in the hippocampus and cortex of elderly APP/PS1 mice.
2.4. Dietary SCFA promote astrocyte-neuron metabolic coupling
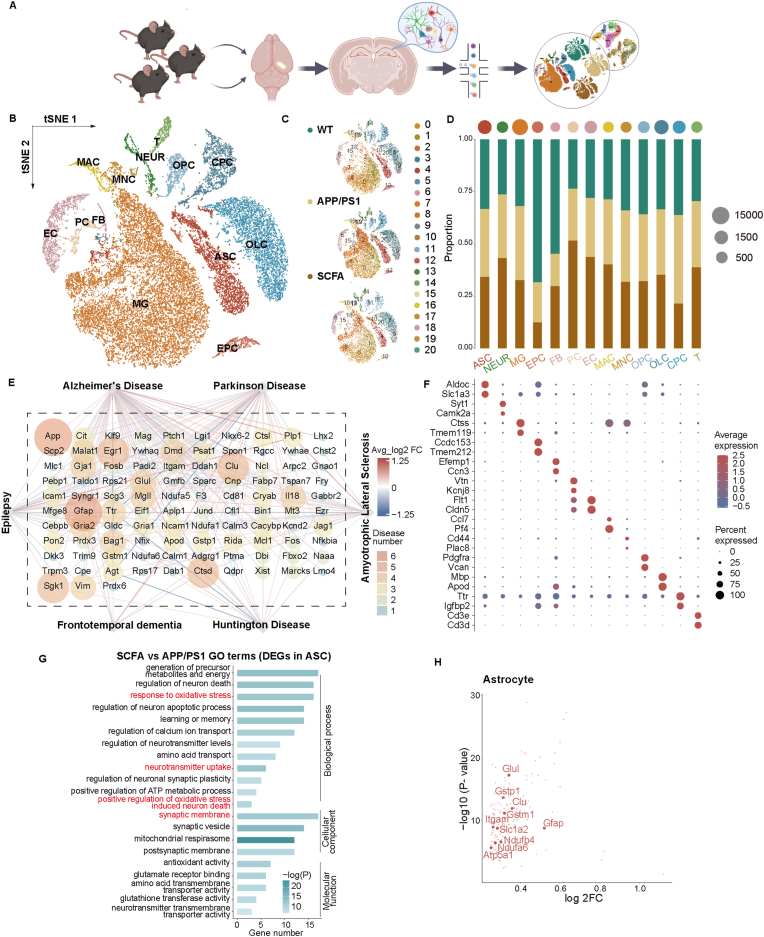
To explore the effects of the dietary SCFA on brain metabolism, especially whether changes in glial cells and neurons contribute to the positive therapeutic effect for AD, we used single-cell RNA sequencing (scRNA seq) technology to analyze the hippocampus of 14-month mice, using three mice per group. We identified 21 clusters of cells and defined 13 cell types, including astrocyte (ASC), neuron (NEUR), microglia (MG), ependymocyte (EPC), fibroblast (FB), pericyte (PC), endothelial cell (EC), macrophage (MAC), monocyte (MNC), oligodendrocyte progenitor cell (OPC), oligodendrocyte cell (OLC), epithelial cell (CPC) and T cell (T) (Fig. 5A–C and F). Given that disease-associated glial cell subpopulations have been implicated in AD and aging [33,34], we therefore investigated the changes of neuron and non-neuron cells (astrocyte and microglia). Our data showed an increase in the proportion of neurons in mice from the SCFA supplementations group, while the astrocyte and microglia remain largely unchanged (Fig. 5D). Interestingly, changes at gene transcription level had been mainly observed in astrocytes (Figs. S4 and S5). Enrichment analysis of differentially expressed genes (DEGs) in astrocytes were also associated with neurodegenerative diseases, particularly AD (Fig. 5E). In addition, SCFA supplementations significantly regulated neurotransmitter uptake, corresponding to pathways related to oxidative stress and synapse, and also upregulation of genes (including Glul, Slc1a2, Gstm1, Gstp1, Clu, Itgam, Atp5a1, Ndufb4, Ndufa6) that involved in astrocyte-neuron metabolic coupling (ANMC) (Fig. 5G and H and S6A-D).
Fig. 5.
Single-cell RNA sequencing to assess the effects of the long-term SCFA supplementation on astrocytes and neurons in APP/PS1 mice. (A) Schematic diagram for the high-throughput single-cell RNA sequencing (scRNA-seq) experiment examining the hippocampus of WT, normal diet APP/PS1, and SCFA diet APP/PS1 mice (created with BioRender.com). (B) t-Distributed stochastic neighbor embedding (t-SNE) showing cells map from hippocampus of 14-month-old mice (10296 cells from 3 WT mice, 12642 cells from 3 normal diet APP/PS1 mice, 8608 cells from 3 SCFA diet APP/PS1 mice with long-term supplementation with SCFA); colored by cell types. ASC: astrocyte, NEUR: neuron, MG: microglia, EPC: ependymocyte, FB: fibroblast, PC: pericyte, EC: endothelial cell, MAC: macrophage, MNC: monocyte, OPC: oligodendrocyte progenitor cell, OLC: oligodendrocyte cell, CPC: epithelial cell, T: T cell. (C) t-SNE plot showing the cell clusters in WT, normal diet APP/PS1, and SCFA diet APP/PS1 mice. 13 cell types were identified with 21 clusters. (D) Bar plot and dot plot showing ratio of cells between WT, normal diet APP/PS1, and SCFA diet APP/PS1 mice and the total number of cells in three groups. (E) Network plot showing an association between differently enrich genes (DEGs) and neurodegenerative disease between normal diet APP/PS1 and SCFA diet APP/PS1 mice. The size and color of each node showed the number of diseases associated with individual genes. (F) Dot plot showing the expression of representative cell markers in all 20 cell clusters between WT, normal diet APP/PS1, and SCFA diet APP/PS1 mice. (G) Bar plot showing the enrichment of Gene Ontology (GO) terms of DEGs in all ASC between WT, normal diet APP/PS1, and SCFA diet APP/PS1 mice (p < 0.05). GO terms sorted from high to bottom with -log(P-value). The color depth of each bar represented the number of DEGs enriched to GO terms. (H) Plot showing log2FC and -log (P-value) of all significant up-regulated DEGs for ASC and between normal diet APP/PS1 and SCFA diet APP/PS1 mice. DEGs associated with astrocyte-neuron metabolic coupling (ANMC) are highlighted (log2FC > 0.25, P-value <0.05). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
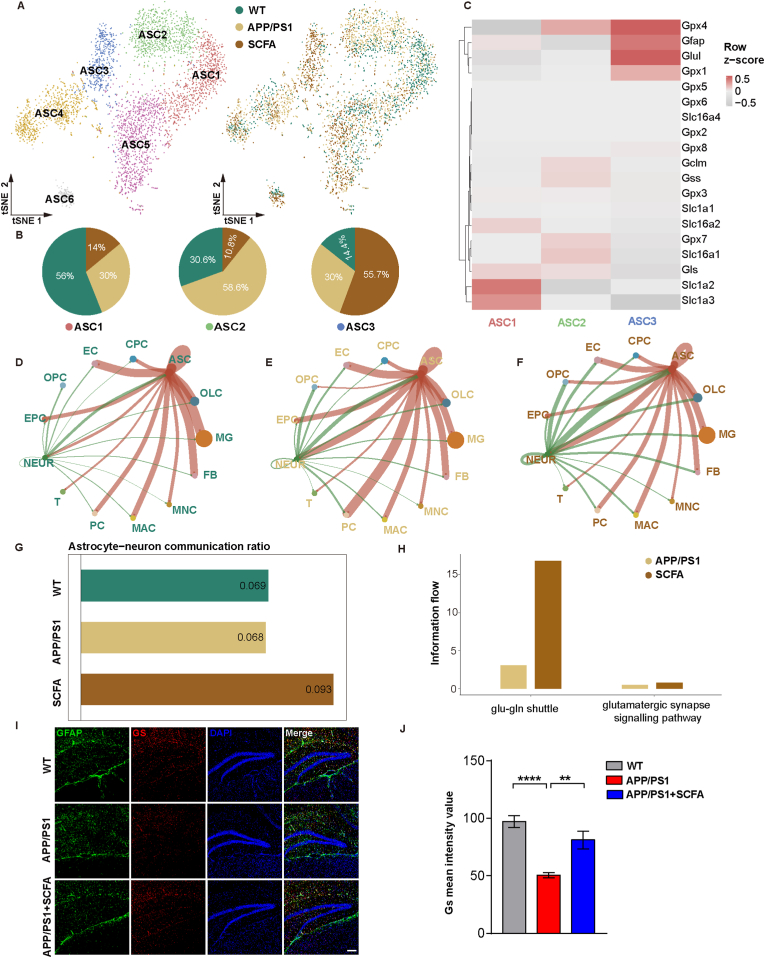
To further explore the detailed changes of astrocytes in the hippocampus, we performed subpopulation delineation and found six different subgroups of astrocytes (ASC1-6) (Fig. 6A). Among these, ASC1 was the dominant group in WT mice (56%), ASC2 in APP/PS1 mice (58.6%), and ASC3 in mice from SCFA supplementations group (55.7%) (Fig. 6B). The transcriptional levels of ANMC-related genes (Glul, Gpx4, Gpx1 and Gfap) were significantly up-regulated in ASC3 subpopulation (Fig. 6C). Further cell-cell interaction analysis showed that SCFA supplementations enhanced the level of astrocyte-neuron communication (Fig. 6D–G). The metabolic coupling of astrocytes with neurons and oxidative damage have been reported to be involved in pathological changes in the AD process [35], while astrocytes form triplex synapses with neurons that attenuate excitatory neurotoxicity by taking up excitatory neuronal transmitters, consistent with our DEGs enrichment analysis results (Fig. 5G and S6A-D). In particular, we noted that SCFA supplementations significantly upregulated the transcription level of glutamine synthetase, which is involved in the astrocyte-neuron glutamate-glutamine shuttle pathway (Fig. 6H). Consistent with this finding, we observed in the APP/PS1 mice, a significant reduction in the expression level of glutamine synthetase, especially in the DG region of the hippocampus compared to that of WT mice, and this reduction can be rescued by SCFA supplementations (Fig. 6I and J). Interestingly, we also previously detected changes in glutamine synthetase activity and expression in astrocytes in vitro, which validated the results of the hippocampal sequencing analysis (Fig. 2). In addition, we examined the changes of genes associated with microglia function, such as activation, secretion and inflammatory responses, and showed that long-term dietary intervention with SCFA did not exacerbate the pro-inflammatory response of microglia (Figs. S9A and B).
Fig. 6.
Dietary SCFA enhance the cell communication of astrocyte-neuron metabolic coupling. (A, B) t-SNE showing 6 different subtypes of ASC cells (A). Left, cells colored by cell subtypes. Right, cells colored by WT, normal diet APP/PS1, and SCFA diet APP/PS1 mice. Sector graph showing the WT mice, APP/PS1 mice and APP/PS1 mice with SCFA supplementation in ASC1, ASC2 and ASC3 (B). (C) Heatmap showing the expression of ANMC-related differential genes in ASC1, ASC2 and ASC3 cell subtypes. Red and gray represent the level of gene Z-scores in the corresponding groups. (D–F) Network plot showing ANMC of ASC and NEUR respective communication with other cell types in WT (D), normal diet APP/PS1 (E), and SCFA diet APP/PS1 mice (F). The thickness of the lines between cells measured the strength of the signal. The red and green lines represented the contents of the ASC or NEUR as ligands. (G) Bar plot showing ratio of ANMC cell communication intensity between ASC and NEUR compared with all cells. (H) Bar plot showing the information flow differences of glutamatergic synapse signaling pathway related and glutamate-glutamine shuttle related cell-cell communication between WT, normal diet APP/PS1, and SCFA diet APP/PS1 mice using CellChat. (I) Effect of SCFA diet on GS levels in the hippocampus DG region of WT, normal diet APP/PS1, and SCFA diet APP/PS1 mice (scale bar, 100 μm). (J) Quantitative analysis of (I) (n = 3 mice; ****p < 0.0001, **p = 0.0023; one-way ANOVA). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Taken together, our data suggest that SCFA supplementations can promote astrocyte-neuron metabolic coupling and upregulate the glutamate-glutamine shuttle, which may contribute to the therapeutic effect of SCFA in AD mice.
3. Discussion
Our understanding of AD pathogenesis still mainly focuses on the CNS, and current clinical drug trials mostly target Aβ and tau but fail to achieve the desired therapeutic effect [36]. Recently, host metabolism change has been implicated in the chronic neurodegenerative disorder of AD, and thus it is also known as type 3 diabetes [[37], [38], [39]]. Accumulating evidence have showed that impaired brain energy metabolism precedes the diagnosis of AD and is present throughout the process, which ultimately creates a vicious cycle leading to neurodegeneration [[40], [41], [42]]. This deficit in energy metabolism is manifested at multiple levels, including mitochondrial damage, impaired ATP production, brain glucose hypometabolism and oxidative damage. In the active brain, neurons rely on oxidative metabolism to consume large amount of energy and are not sufficiently capable of resisting oxidative damage on their own [18]. Astrocytes, as one of the glial cells, are responsible for maintaining neuronal physiological activities including the timely removal of excessive excitatory neurotransmitters from the synaptic gap, high rates of glycolysis to provide lactate to neurons and the glutamate-glutamine cycle to provide antioxidant substrates to neurons, and these metabolic couplings support the role of astrocytes in brain energy metabolism [18].
Although several studies have confirmed the existence of multiple effects on the regulation of AD by SCFA [22,23,[43], [44], [45]], the mechanisms of a long-term SCFA diet to the brain energy metabolism remain unclear. We therefore administered SCFA drinking water feeding to APP/PS1 mice for up to 9 months during early aging. The results showed that a long-term dietary SCFA supplementations significantly improve the cognitive learning and spatial memory abilities, and reduce amyloid plaque deposition and abnormal phosphorylation of tau, in hippocampus and cortex of elderly APP/PS1 mice. We further found that SCFA enhanced astrocyte-neuron communication mainly by modulating astrocytes through scRNA seq data of mice hippocampus, which is consistent with the findings in the astrocyte-neuron co-culture system. In addition, given that metabolism of dietary fibre by gut microbiota is an important source of SCFA, we compared the gut microbial composition characteristics between APP/PS1 and WT mice at two time points of age, 7- and 14-month. In particular, at the age of 7-month, by 16S sequence and analysis, the APP/PS1 mice has lower bacterial abundance in families Ruminococcaceae, Clostridiaceae, Veillonellaceae and order Lachnospira, which are known for producing SCFA, than that in the WT mice. While at the age of 14-month, the APP/PS1 mice gut microbiome has much lower abundance of Prevotellaceae (Fig. S7). Admittedly, the APP/PS1 mice has potentially lower SCFA supplies from gut microbiome, which is consistent with previous reports [46]. The dysbiosis of the APP/PS1 mice gut microbiota has been effectively improved by SCFA diet, with an increase in the abundance of SCFA producers including Akkermansia and Clostridium_XIVb.
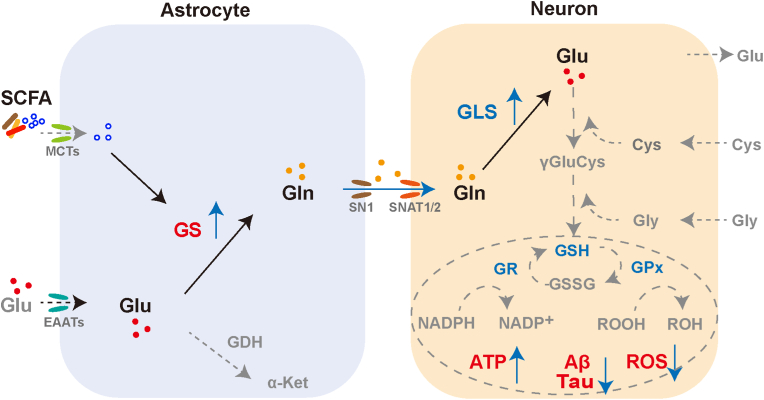
Glutamate is an important excitatory neurotransmitter whose levels in the excitatory synaptic gap are strictly regulated by sodium-dependent excitatory amino acid transporters (EAATs) to prevent excitotoxicity. As a protector of neurons, astrocytes predominantly express EAAT1 (glutamate/aspartate transporter (GLAST), also known as Slc1a3), EAAT2 (glutamate transporter-2 (GLT-1), also known as Slc1a2) and take up excess glutamate in the synaptic gap in time [47]. By analyzing the scRNA seq data, we found that the expression levels of Slc1a2 and Slc1a3 were significantly upregulated in astrocytes of APP/PS1mice with SCFA diet, compared with the normal diet group (Fig. S8A). The Slc38 gene family of sodium-coupled neutral amino acid transporters (SNAT) plays an important role in the transport of glutamine from astrocytes to neurons. The main regulators of glutamine efflux in astrocytes are SNAT3 (also known as Slc38a3 and SN1) and SNAT5 (also known as Slc38a5 and SN2), while SNAT1(also known as Slc38a1) and SNAT2 (also known as Slc38a2) are responsible for regulating glutamine uptake by neurons [[48], [49], [50]]. Our results showed that SCFA treatment did not affect the expression of Slc38a3 in astrocytes and Slc38a2 in neurons, but upregulated the expression of neuronal Slc38a1 (Figs. S8B and C). Taken together, SCFA accelerated glutamate uptake and conversion to glutamine but did not affect glutamine efflux in astrocyte, and promoted glutamine uptake by neurons (Fig. 7).
Fig. 7.
Schematic diagram of the model for SCFA-regulated astrocyte-neuron glutamate-glutamine shuttle.
An imbalance between reactive oxygen species production and antioxidant defences is a typical trigger of neuronal damage and death. Astrocytes can efficiently supply neuron with antioxidant substrates to rescue disorders of brain energy metabolism in AD. Astrocytes take up excess glutamate in the synaptic gap, convert it to glutamine via glutamine synthetase and shuttle it to the neurons, where glutamine is reconverted to glutamate by glutaminases. Glutamate in turn is involved in glutathione synthesis and exerts antioxidant effects in neurons. Given that neurons lack the carboxylase for the de novo synthesis of the glutamate, the involvement of astrocytes compensates for the shortcomings of neurons. Our study found that SCFA promote glutamate-glutamine cycle by upregulating astrocyte GS, and consequently countermeasure mitochondrial dysfunction, oxidative stress damage and AD-like lesions. We aim to provide new perspectives on AD prevention and treatment strategies from the regulation of brain energy metabolism (Glu, glutamate; Gln, glutamine; GS, glutamine synthetase; GLS, glutaminase; Cys, cysteine; Gly, glycine; GSH, glutathione; GSSG, glutathione oxidized; GR, glutathione reductase; GPx, glutathione peroxidase; MCTs, monocarboxylate transporters; EAATs, excitatory amino acid transporters; SN1 and SNAT1/2, sodium-coupled neutral amino acid transporters; GDH, glutamate dehydrogenase; α-Ket, α-Ketoglutarate).
Currently, six SCFA membrane receptors, GPR41 (also known as FFAR3), GPR42, GPR43 (also known as FFAR2), GPR109A, GPR164, OLFR78, have been identified, and among them, FFAR2, FFAR3 and GPR109A have been more widely studied. FFAR2 is mainly expressed in the vascular system, immune cells, adipose tissue and intestinal endocrine cells. FFAR3 is expressed in the kidney, colon and blood vessels. GPR109A is expressed in adipocytes, immune cells and intestinal epithelial cells [20,51]. We did not detect the expression of six membrane SCFA receptors in astrocytes by single-cell sequencing of hippocampus in mice, but the SCFA transporters Slc16a1, Slc16a3 and Slc16a7 were identified (Fig. S8D). We proposed that the SCFA were uptaked by the SCFA transporters in astrocyte.
To assess the effect of SCFA on the involvement of glutamine metabolism in the production of GSH to counteract oxidative damage in neurons, in the astrocyte-neuron co-culture system, we detected that SCFA significantly alleviated Aβ-induced abnormalities of GSH levels, whereas MSO abolished this effect in neurons (Fig. S8E). Furthermore, SCFA had no effect on the expression levels of glutathione synthase and glutamate-cysteine ligase in neurons (Fig. S8F), and mainly affected neuronal ATP levels and ROS levels (Fig. 2H and I). Previous studies have showed that SCFA entered the tricarboxylic acid cycle to generate ATP for cellular energy, and intracellular SCFA can inhibit histone deacetylases and contribute to the recovery of mitochondrial function and cognitive capacity [20,21,25,26]. Therefore, the metabolism of SCFA would be involved in protective effects on neuronal cells. According to our and previous findings, we suggest that SCFA treatment may promote a synergic protective response.
Otherwise, the SCFA effects were determined by a quite complicated process. For glutamate-related metabolism in astrocytes (Fig. 7), it can be converted to glutamine by the glutamine synthetase, and also be catalyzed by glutamate dehydrogenase to produce α-ketoglutarate which participates in the TCA cycle and ultimately produces ATP [52]. GS activity is associated with the regulation of adenylation and the involvement of metal ions. The rate of adenylation depends on the ratio of glutamine to α-ketoglutarate [53]. It remains unclear how acetate, propionate and butyrate or mixtures affect the levels of α-ketoglutarate and the intracellular uptake of metal ions.
For transcription factors involved in the SCFA-induced GS expression in astrocytes, previous study showed that the forkhead box O (FoxO) family of transcription factors plays a key role in the aging process, and in particular FoxO1 and FoxO3 received widespread attention in metabolic disorders such as diabetes, cancer and neurodegenerative diseases [54]. Fluteau et al. found that FoxO3 upregulated GS expression in astrocytes in response to oxidative stress and modulated DNA damage to exert neuroprotection [55]. In addition, GS was transcriptionally regulated by the phosphoinositide-3-kinase (PI3K)-Akt-FoxO pathway [56]. Cohort studies have shown that increased levels of AD pathology were closely associated with reduced glutamate uptake transporters (EAATs) and downregulation of the PI3K-Akt pathway in astrocytes [57,58]. Taken together, we suggest that FoxO is a key transcription factor for SCFA to upregulate GS in astrocytes to mitigate neuronal oxidative damage, and the PI3K-Akt-FoxO pathway may also be a target for future exploration of how SCFA regulate GS.
Our data support the contribution of a long-term SCFA diet to the metabolic interactions between astrocytes and neurons during aging process, which highlights an exciting strategy of starting dietary therapy early in aging before the onset of brain energy deficiency. Admittedly, drug development targeting abnormalities in brain energy metabolism during AD process would emerge as a new research field for disease intervention. Of note, our findings were in direct contradiction to the deteriorating effects of short-term administration of SCFA in aged APP/PS1 mice [43]. Given that these deteriorating effects are mostly involving pro-inflammatory responses induced by microglial cells in brain regions of aged APP/PS1 mice [59], our data revealed that most of the key genes related to inflammatory responses were not significantly upregulated in microglia in the APP/PS1 mice with a long-term SCFA diet. This difference might be resulting from the fact that we fed the mice with SCFA at different ages. Otherwise, we cannot rule out the possibility that other favorable driving forces may exist during aging in response to long-term SCFA diet.
Our study provides valuable insight into the participation of diet in host physiological processes including brain energy metabolism and will pave the way for the development of dietary interventions and new drug in AD treatment by mobilizing astrocyte-neuron glutamate transport pathways. Further studies are still required to elucidate the mechanisms for SCFA regulation of astrocytic GS and the synergistic features of multiple metabolic couplings in the AD process.
Author contributions
Y.S. conceived and performed the study idea, gathered and analyzed the data, and wrote the manuscript. H.Z. conducted the data analysis of sc-RNA seq. X. Z performed 16S rRNA analysis. W.W contributed to the LC-MS of glutamine. Y.C and Z. C contributed to the final manuscript. J.W. and Q.W. wrote manuscript and study supervision. Yi Shi conceived and supervised the project, and wrote the manuscript. All authors read and approved the final manuscript.
Declaration of competing interest
The authors declare no competing interests.
Acknowledgements
We appreciate the technical support staff at the Institutional Center for Shared Technologies and Facilities of Institute of Psychology, Chinese Academy of Sciences for assistance with animal experiments and behavioral tests. We thank X. Z (Institute of Microbiology, CAS) for technical support with confocal imaging. This study was supported by the Strategic Priority Research Program of Chinese Academy of Sciences, China (XDB29010000 and XDB29020000). Yi Shi is also supported the Youth Innovation Promotion Association of Chinese Academy of Sciences, China (Y201921).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2023.102690.
Contributor Information
Qihui Wang, Email: wangqihui@im.ac.cn.
Jun Wang, Email: junwang@im.ac.cn.
Yi Shi, Email: shiyi@im.ac.cn.
Appendix A. Supplementary data
The following is/are the supplementary data to this article.
Data availability
Data will be made available on request.
References
- 1.Mucke L. Alzheimer's disease. Nature. 2009;461:895–897. doi: 10.1038/461895a. [DOI] [PubMed] [Google Scholar]
- 2.Villemagne V.L., Dore V., Burnham S.C., Masters C.L., Rowe C.C. Imaging tau and amyloid-beta proteinopathies in Alzheimer disease and other conditions. Nat. Rev. Neurol. 2018;14:225–236. doi: 10.1038/nrneurol.2018.9. [DOI] [PubMed] [Google Scholar]
- 3.Lin M.T., Beal M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 4.Kapogiannis D., Mattson M.P. Disrupted energy metabolism and neuronal circuit dysfunction in cognitive impairment and Alzheimer's disease. Lancet Neurol. 2011;10:187–198. doi: 10.1016/S1474-4422(10)70277-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amieva H., et al. The 9 year cognitive decline before dementia of the Alzheimer type: a prospective population-based study. Brain. 2005;128:1093–1101. doi: 10.1093/brain/awh451. [DOI] [PubMed] [Google Scholar]
- 6.Jack C.R., Jr., et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Citron M. Alzheimer's disease: strategies for disease modification. Nat. Rev. Drug Discov. 2010;9:387–398. doi: 10.1038/nrd2896. [DOI] [PubMed] [Google Scholar]
- 8.Dizdaroglu M., Coskun E., Jaruga P. Measurement of oxidatively induced DNA damage and its repair, by mass spectrometric techniques. Free Radic. Res. 2015;49:525–548. doi: 10.3109/10715762.2015.1014814. [DOI] [PubMed] [Google Scholar]
- 9.Halliwell B., Gutteridge J.M. Oxford university press; USA: 2015. Free Radicals in Biology and Medicine. [Google Scholar]
- 10.Butterfield D.A., Boyd-Kimball D. Oxidative stress, amyloid-β peptide, and altered key molecular pathways in the pathogenesis and progression of Alzheimer's disease. J Alzheimers Dis. 2018;62:1345–1367. doi: 10.3233/JAD-170543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao D., et al. A dual-targeted multifunctional nanoformulation for potential prevention and therapy of Alzheimer's disease. Innovation. 2021;2 doi: 10.1016/j.xinn.2021.100160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee J., et al. SIRT3 deregulation is linked to mitochondrial dysfunction in Alzheimer's disease. Aging Cell. 2018;17 doi: 10.1111/acel.12679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cunnane S.C., et al. Can ketones help rescue brain fuel supply in later life? Implications for cognitive health during aging and the treatment of Alzheimer's disease. Front. Mol. Neurosci. 2016;53 doi: 10.3389/fnmol.2016.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson H., Pagano G., Politis M. Dementia spectrum disorders: lessons learnt from decades with PET research. J. Neural. Transm. 2019;126:233–251. doi: 10.1007/s00702-019-01975-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ioannou M.S., et al. Neuron-astrocyte metabolic coupling protects against activity-induced fatty acid toxicity. Cell. 2019;177:1522–1535. doi: 10.1016/j.cell.2019.04.001. e1514. [DOI] [PubMed] [Google Scholar]
- 16.Magistretti P.J., Allaman I. Lactate in the brain: from metabolic end-product to signalling molecule. Nat. Rev. Neurosci. 2018;19:235–249. doi: 10.1038/nrn.2018.19. [DOI] [PubMed] [Google Scholar]
- 17.Barros L.F., Brown A., Swanson R.A.G. Lia in brain energy metabolism: a perspective. Glia. 2018;66:1134–1137. doi: 10.1002/glia.23316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bélanger M., Allaman I., Magistretti P.J. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metabol. 2011;14:724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 19.Cummings J., Pomare E., Branch W., Naylor C., Macfarlane G. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dalile B., Van Oudenhove L., Vervliet B., Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019;16:461–478. doi: 10.1038/s41575-019-0157-3. [DOI] [PubMed] [Google Scholar]
- 21.Govindarajan N., Agis-Balboa R.C., Walter J., Sananbenesi F., Fischer A. Sodium butyrate improves memory function in an Alzheimer's disease mouse model when administered at an advanced stage of disease progression. J Alzheimers Dis. 2011;26:187–197. doi: 10.3233/JAD-2011-110080. [DOI] [PubMed] [Google Scholar]
- 22.Fernando W., et al. Sodium butyrate reduces brain amyloid-β levels and improves cognitive memory performance in an Alzheimer's disease transgenic mouse model at an early disease stage. J Alzheimers Dis. 2020;74:91–99. doi: 10.3233/JAD-190120. [DOI] [PubMed] [Google Scholar]
- 23.Jiang Y., Li K., Li X., Xu L., Yang Z. Sodium butyrate ameliorates the impairment of synaptic plasticity by inhibiting the neuroinflammation in 5XFAD mice. Chem. Biol. Interact. 2021;341 doi: 10.1016/j.cbi.2021.109452. [DOI] [PubMed] [Google Scholar]
- 24.Bergman E. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 1990;70:567–590. doi: 10.1152/physrev.1990.70.2.567. [DOI] [PubMed] [Google Scholar]
- 25.Cani P.D., et al. Microbial regulation of organismal energy homeostasis. Nat Metab. 2019;1:34–46. doi: 10.1038/s42255-018-0017-4. [DOI] [PubMed] [Google Scholar]
- 26.Bogie J.F., Haidar M., Kooij G., Hendriks J.J. Fatty acid metabolism in the progression and resolution of CNS disorders. Adv. Drug Deliv. Rev. 2020;159:198–213. doi: 10.1016/j.addr.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Serpa J., et al. Butyrate-rich colonic microenvironment is a relevant selection factor for metabolically adapted tumor cells. J. Biol. Chem. 2010;285:39211–39223. doi: 10.1074/jbc.M110.156026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donohoe D.R., et al. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metabol. 2011;13:517–526. doi: 10.1016/j.cmet.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fillier T.A., et al. Brief exposure of neuronal cells to levels of SCFAs observed in human systemic circulation impair lipid metabolism resulting in apoptosis. Sci. Rep. 2022;12:1–16. doi: 10.1038/s41598-022-18363-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moretti M., et al. Behavioral and neurochemical effects of sodium butyrate in an animal model of mania. Behav. Pharmacol. 2011;22:766–772. doi: 10.1097/FBP.0b013e32834d0f1b. [DOI] [PubMed] [Google Scholar]
- 31.De Vadder F., et al. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156:84–96. doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 32.Martorell A.J., et al. Multi-sensory gamma stimulation ameliorates alzheimer's-associated pathology and improves cognition. Cell. 2019;177:256–271 e222. doi: 10.1016/j.cell.2019.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olah M., et al. Single cell RNA sequencing of human microglia uncovers a subset associated with Alzheimer's disease. Nat. Commun. 2020;11:6129. doi: 10.1038/s41467-020-19737-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Habib N., et al. Disease-associated astrocytes in Alzheimer's disease and aging. Nat. Neurosci. 2020;23:701–706. doi: 10.1038/s41593-020-0624-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun Y., et al. Modulation of the astrocyte-neuron lactate shuttle system contributes to neuroprotective action of fibroblast growth factor 21. Theranostics. 2020;10:8430. doi: 10.7150/thno.44370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cummings J., Lee G., Ritter A., Sabbagh M., Zhong K. Alzheimer's disease drug development pipeline: 2019. Alzheimers Dement (N Y) 2019;5:272–293. doi: 10.1016/j.trci.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson E.C., et al. Large-scale proteomic analysis of Alzheimer's disease brain and cerebrospinal fluid reveals early changes in energy metabolism associated with microglia and astrocyte activation. Nat. Med. 2020;26:769–780. doi: 10.1038/s41591-020-0815-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pentz R., Iulita M.F., Ducatenzeiler A., Bennett D.A., Cuello A.C. The human brain NGF metabolic pathway is impaired in the pre-clinical and clinical continuum of Alzheimers disease. Mol. Psychiatr. 2020:1–15. doi: 10.1038/s41380-020-0797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suzanne M. Type 3 diabetes is sporadic Alzheimer׳ s disease: mini-review. Eur. Neuropsychopharmacol. 2014;24:1954–1960. doi: 10.1016/j.euroneuro.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Butterfield D.A., Halliwell B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019;20:148–160. doi: 10.1038/s41583-019-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cunnane S.C., et al. Brain energy rescue: an emerging therapeutic concept for neurodegenerative disorders of ageing. Nat. Rev. Drug Discov. 2020;19:609–633. doi: 10.1038/s41573-020-0072-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Croteau E., et al. A cross-sectional comparison of brain glucose and ketone metabolism in cognitively healthy older adults, mild cognitive impairment and early Alzheimer's disease. Exp. Gerontol. 2018;107:18–26. doi: 10.1016/j.exger.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 43.Colombo A.V., et al. Microbiota-derived short chain fatty acids modulate microglia and promote Aβ plaque deposition. Elife. 2021;10 doi: 10.7554/eLife.59826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Erny D., et al. Microbiota-derived acetate enables the metabolic fitness of the brain innate immune system during health and disease. Cell Metabol. 2021;33:2260–2276. doi: 10.1016/j.cmet.2021.10.010. e2267. [DOI] [PubMed] [Google Scholar]
- 45.Liu J., et al. Anti-neuroinflammatory effect of short-chain fatty acid acetate against alzheimer's disease via upregulating GPR41 and inhibiting ERK/JNK/NF-κB. J. Agric. Food Chem. 2020;68:7152–7161. doi: 10.1021/acs.jafc.0c02807. [DOI] [PubMed] [Google Scholar]
- 46.Seo D.-O., Holtzman D.M. Gut microbiota: from the forgotten organ to a potential key player in the pathology of Alzheimer's disease. J Gerontol A Biol Sci Med Sci. 2020;75:1232–1241. doi: 10.1093/gerona/glz262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rothstein J.D., et al. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 48.Bröer S., Brookes N. Transfer of glutamine between astrocytes and neurons. J. Neurochem. 2001;77:705–719. doi: 10.1046/j.1471-4159.2001.00322.x. [DOI] [PubMed] [Google Scholar]
- 49.Chaudhry F.A., et al. Glutamine uptake by neurons: interaction of protons with system a transporters. J. Neurosci. 2002;22:62–72. doi: 10.1523/JNEUROSCI.22-01-00062.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hamdani E.H., Gudbrandsen M., Bjørkmo M., Chaudhry F.A. The system N transporter SN2 doubles as a transmitter precursor furnisher and a potential regulator of NMDA receptors. Glia. 2012;60:1671–1683. doi: 10.1002/glia.22386. [DOI] [PubMed] [Google Scholar]
- 51.Mishra S.P., Karunakar P., Taraphder S., Yadav H. Free fatty acid receptors 2 and 3 as microbial metabolite sensors to shape host health: pharmacophysiological view. Biomedicines. 2020;8:154. doi: 10.3390/biomedicines8060154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mahmoud S., Gharagozloo M., Simard C., Gris D. Astrocytes maintain glutamate homeostasis in the CNS by controlling the balance between glutamate uptake and release. Cells. 2019;8:184. doi: 10.3390/cells8020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jayakumar A.R., Norenberg M.D. Glutamine synthetase: role in neurological disorders. Adv Neurobiol. 2016:327–350. doi: 10.1007/978-3-319-45096-4_13. [DOI] [PubMed] [Google Scholar]
- 54.Hu W., et al. Roles of forkhead box O (FoxO) transcription factors in neurodegenerative diseases: a panoramic view. Prog. Neurobiol. 2019;181 doi: 10.1016/j.pneurobio.2019.101645. [DOI] [PubMed] [Google Scholar]
- 55.Fluteau A., et al. The nuclear retention of transcription factor FOXO3a correlates with a DNA damage response and increased glutamine synthetase expression by astrocytes suggesting a neuroprotective role in the ageing brain. Neurosci. Lett. 2015;609:11–17. doi: 10.1016/j.neulet.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Der Vos K.E., et al. Modulation of glutamine metabolism by the PI (3) K–PKB–FOXO network regulates autophagy. Nat. Cell Biol. 2012;14:829–837. doi: 10.1038/ncb2536. [DOI] [PubMed] [Google Scholar]
- 57.Simpson J., et al. Astrocyte phenotype in relation to Alzheimer-type pathology in the ageing brain. Neurobiol. Aging. 2010;31:578–590. doi: 10.1016/j.neurobiolaging.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 58.Simpson J.E., et al. Microarray analysis of the astrocyte transcriptome in the aging brain: relationship to Alzheimer's pathology and APOE genotype. Neurobiol. Aging. 2011;32:1795–1807. doi: 10.1016/j.neurobiolaging.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 59.Babcock A.A., et al. Cytokine-producing microglia have an altered beta-amyloid load in aged APP/PS1 Tg mice. Brain Behav. Immun. 2015;48:86–101. doi: 10.1016/j.bbi.2015.03.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.