Abstract
Colorectal cancer (CRC) is the most common digestive malignancy across the world. Its first-line treatments applied in the routine clinical setting include surgery, chemotherapy, radiotherapy, targeted therapy, and immunotherapy. However, resistance to therapy has been identified as the major clinical challenge that fails the treatment method, leading to recurrence and distant metastasis. An increasing number of studies have been attempting to explore the underlying mechanisms of the resistance of CRC cells to different therapies, which can be summarized into two aspects: (1) The intrinsic characters and adapted alterations of CRC cells before and during treatment that regulate the drug metabolism, drug transport, drug target, and the activation of signaling pathways; and (2) the suppressive features of the tumor microenvironment (TME). To combat the issue of therapeutic resistance, effective strategies are warranted with a focus on the restoration of CRC cells’ sensitivity to specific treatments as well as reprogramming impressive TME into stimulatory conditions. To date, nanotechnology seems promising with scope for improvement of drug mobility, treatment efficacy, and reduction of systemic toxicity. The instinctive advantages offered by nanomaterials enable the diversity of loading cargoes to increase drug concentration and targeting specificity, as well as offer a platform for trying the combination of different treatments to eventually prevent tumor recurrence, metastasis, and reversion of therapy resistance. The present review intends to summarize the known mechanisms of CRC resistance to chemotherapy, radiotherapy, immunotherapy, and targeted therapy, as well as the process of metastasis. We have also emphasized the recent application of nanomaterials in combating therapeutic resistance and preventing metastasis either by combining with other treatment approaches or alone. In summary, nanomedicine is an emerging technology with potential for CRC treatment; hence, efforts should be devoted to targeting cancer cells for the restoration of therapeutic sensitivity as well as reprogramming the TME. It is believed that the combined strategy will be beneficial to achieve synergistic outcomes contributing to control and management of CRC in the future.
Keywords: Colorectal cancer, Therapeutic resistance, Nanotechnology, Drug delivery system, Combined treatment
Core Tip: Mechanisms of Colorectal cancer (CRC) cell resistance can be attributed to tumoral and environmental factors, the former of which includes gene expression alteration, signaling pathway activation, metabolic rewiring, and the later refers to complicated adaptions regarding cancer associated fibroblast, immune cells, hypoxic conditions. Taking efforts to conquer therapeutic resistance is imperative to improve CRC patients` survival. Nanotechnology possesses distinct advantages to increase specificity of treatment and realize codelivery of multiple drugs, which facilitates to restore sensitivity to antitumor therapy, and modulate suppressive tumor microenvironment to stimulatory environment.
INTRODUCTION
Colorectal cancer (CRC) is the most common malignancy of the digestive tract, accounting for the death of 0.9 million people across the world in 2020 alone[1]. Surgery, chemotherapy, radiotherapy, targeted therapy, and immunotherapy are among the present first-line strategies applied to treat CRC patients and control their recurrence. Surgery is accepted as the primary treatment for CRC, especially for early stage CRC, as it eradiates the local lesions thoroughly in a timely manner[2]. Five-Flurouracil (5-FU)- and oxaliplatin-based chemotherapy contribute to the control of local recurrence and metastasis of CRC after surgery. As for advanced CRC, radiotherapy and neoadjuvant chemotherapy are important modalities that have been reported to contribute to prolonging the patients’ survival[3,4]. In addition, cetuximab and immunocheckpoint inhibitor (ICI)-based therapy can be administrated to EGFR-overexpressed CRC or microsatellite instability (MSI)-subtype CRC so as to achieve greater survival benefits[5,6]. However, despite the availability of comprehensive treatment against CRC, resistance to therapy and metastasis continue to be reported during or after the treatment, often resulting in relapse or treatment failure.
Chemotherapy resistance is a common cause of CRC treatment failure, and the intrinsic and acquired resistance of tumor cells have been implicated as the major causes[7]. Aberrant metabolism and change in the drug targets have generally been reported to be responsible for the 5-FU resistance of CRC cells developed during the treatment duration[8,9]. The development of resistance to radiotherapy is usually related to hypoxia and the adaption of cancer stem cells (CSC) that play a pivotal role in the regulation of survival and proliferation of CRC[10]. In addition, the tumor microenvironment (TME) is involved in the revolution and development of tumor cells to resist attacks from drugs and immune cells[11]. Many efforts have been made toward enhancing the treatment efficacy after resistance is developed, such as by increasing the drug dosage or combining multiple treatments. However, the improvements in response and the overall survival rate remain unsatisfactory due to the issues of increased toxicity and poor tolerance. Consequently, more effective drug-delivery methods and therapeutic strategies are urgently warranted to overcome the barrier of the suppressive microenvironment as well as to restore the sensitivity of CRC cells to the prescribed treatment.
Thanks to the unique advantages offered by nanotechnology, it has drawn the great attention of researchers and clinicians in their quest to conquer the issue of treatment resistance of CRC with its valuable potential. Drug-delivery systems based on nanomaterials can simultaneously carry a variety of components and increase the selectivity of the systemic treatment and the local drug concentration through active and passive targeting effects[12-14]. This approach not only delivers cytotoxic drugs but also delivers nucleic acid molecules, thereby realizing the combined application of chemotherapy, immunotherapy, gene therapy, and other treatments[15-17]. In addition, several nanomaterials possess anticancer potential or can hence be utilized as radiotherapy sensitizers and they generate a photothermal or photodynamic effect that can facilitate tumor control[18,19]. When used either intraoperatively or postoperatively, nanomaterial-based treatment can be applied to remodel the local microenvironment and activate the innate and adaptive immune responses toward controlling tumor growth and metastasis, which, in turn, generate immune-memory effects called immunomodulation[20-22]. Collectively, nanotechnology is expected to solve the treatment resistance of CRC as a valuable alteration to realize the effective control of tumor recurrence and metastasis. In this review, we have illustrated the mechanisms of therapeutic resistance of CRC cells to various treatments, including both tumoral and environmental factors (Figure 1). Meanwhile, recent advancements in nanotechnology utilized in CRC treatment aimed at restoring tumor sensitivity and reversing resistance will be further introduced, along with the challenges and perspectives for nanotechnology-based CRC treatment.
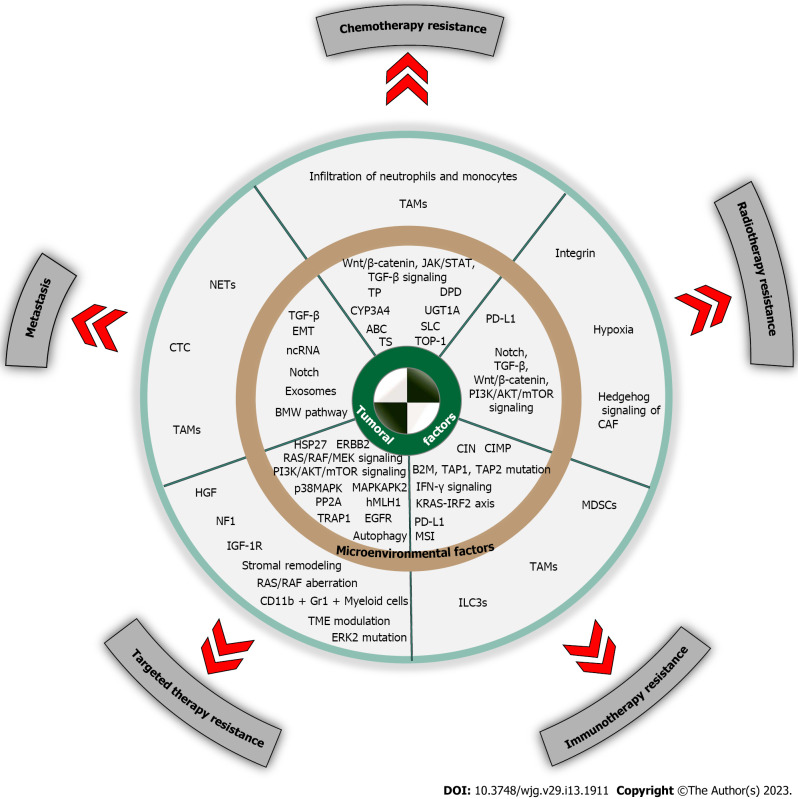
Figure 1.
Schematic illustration of mechanisms of colorectal cancer cell resistance to chemotherapy, radiotherapy, immunotherapy, targeted therapy, and metastasis. ABC: ATP binding cassette; B2M: β2-microglobulin; CAFs: Cancer associated fibroblasts; CIMP: CpG island methylator phenotype; CIN: Chromosomal instability; CTC: Circulating tumor cell; CYP3A4: Cytochrome P450 3A4; DPD: Dihydropyrimidine dehydrogenase; EGFR: Epidermal growth factor receptor; EMT: Epithelial-mesenchymal transition; ERBB2: Erb-b2 receptor tyrosine kinase 2; ERK2: Extracellular regulated protein kinases; HGF: Hepatocyte growth factor; hMLH1: Human MutL homolog 1; HSP27: Heat shock protein 27; IGF-1R: Insulin-like growth factor 1 receptor; ILC3s: Group 3 innate lymphoid cells; MAPKAPK2: Mitogen-activated protein; MDSCs: Myeloid-derived suppressive cells; MSI: Microsatellite instability; NETs: Neutrophil extracellular traps; NF1: Neurofibromin 1; p38MAPK: p38 mitogen-activated protein kinase; PP2A: Protein phosphatase 2A; SLC: Solute carrier; TAP1: Transporter 1; TAP2: Transporter 2; TAMs: Tumor associated macrophages; TME: Tumor microenvironment; TOP-1: Topoisomerase 1; TP: Thymine phosphorylase; TRAP1: TNF receptor associated protein 1; TS: Thymidylate synthase; UGT1A: Uridine diphosphate glucuronosyltransferase 1A.
MECHANISMS OF THERAPEUTIC RESISTANCE AND METASTASIS
Chemotherapy resistance
Fluorouracil, oxaliplatin, and irinotecan are the three main reagents used for CRC adjuvant chemotherapy, which exert antitumor effects through interaction with DNA. However, during the treatment, tumor cells adapt several mechanisms to evade the attack of the treatment agent. Generally, the tumoral mechanism of developing resistance can be categorized into 4 aspects: alterations in the pharmacokinetics and metabolism, transport process, and targets of chemotherapeutics as well as aberrant activation of oncogenic/bypass signaling (Figure 1).
In the case of pharmacokinetics and drug metabolism, reduced concentrations, decreased activation, and increased inactivation of drugs in tumor site, all contribute to the adaption of resistance. Efforts have been focused on investigating the importance of pharmacogenomics on intrinsic and acquired resistance in CRC. Polymorphism of genes encoding critical enzymes during drug absorption, distribution, metabolism and detoxication, has been identified which will facilitate targeted personalized therapy, such as UDP-glucuronosyltransferases (UGT1As) and glutathione-S-transferase (GSTs)[23]. The transformation of capecitabine (CAP) to 5-FU is essential for direct cytotoxicity, which is mediated by thymine phosphorylase (TP)[24]. According to a past report, tumor cells apply epigenetic alterations to downregulate the TP expression, which leads to the development of drug resistance to CAP, albeit it can be reversed by DNA methyltransferase[25]. Similarly, changes in the activity of the key enzymes of 5-FU and irinotecan were evaluated by CRC cells affecting drug sensitivity[8,9,26,27]. Moreover, the inactivation of drug metabolites facilitates the development of drug resistance by CRC cells. Considering that dihydropyrimidine dehydrogenase (DPD) catabolizes 5-FU into an inactive product, the investigation of CRC patients revealed that a high level of DPD expression was significantly related to the resistance to 5-FU[28]. Irinotecan and its active form SN38 can be converted into an inactive form by several enzymes, such as cytochrome P450 (CYP) 3A4 and uridine diphosphate glucuronosyltransferase 1A, and therapeutic strategies targeting these enzymes have been proposed to effectively reverse irinotecan resistance[29,30].
Changes in drug transport are another important factor for drug resistance. Membrane transport pumps can transport chemotherapeutic drugs or molecular targeted drugs out of the cells, thereby reducing the intracellular concentrations and leading to the development of multidrug resistance (MDR). Membrane transporters mainly include 2 families: ATP binding cassette (ABC) and solute carrier transporters. ABC transporters are the most famous and the main membrane transporter for MDR, executing an important role in CRC resistance to chemotherapy. P-glycoprotein (P-gp) is the first identified ABC superfamily member, and its upregulated expression has been implicated in the development of MDR leading to chemotherapy failure in CRC[31,32]. To date, several preclinical studies have demonstrated that natural inhibitors targeting ABCs or antisense oligonucleotide and ncRNAs aiming to downregulate the P-gp expression possess the therapeutic potential to reverse the resistance of CRC cells to oxaliplatin and 5-FU[33-36].
Alterations in the drug targets play a role in mediating the generation of drug resistance. Thymidylate synthase (TS) is the main target of 5-FU in destroying DNA replication, and its expression plays a crucial role in the development of CRC resistance to 5-FU[28]. Efforts to interfere with 5-FU-mediated TS inhibition are expected to disrupt the development of drug resistance. Folic acid (FA) enhances the cytotoxicity of 5-FU through the inhibition of TS both in vitro and in vivo[37,38]. Topoisomerase 1 (TOP-1) is the main target of irinotecan, and its mutation or downregulation is responsible for CRC resistance, as reduced TOP-1 binding to DNA also contributes to irinotecan resistance[27,39]. Past evidence suggests that Y-box binding protein 1 (YB-1) can improve the sensitivity of irinotecan through its direct interaction with TOP-1 toward promoting intracellular TOP-1 activity[40].
As for oncogenic/bypass signaling, it has been demonstrated that the sustained activation of Wnt/β-catenin, JAK/STAT, and TGF-β contributes to drug resistance of CRC. Wnt/β-catenin signaling is a well-known evolutionarily conserved pathway endowing tumor cells with sustained growth and is responsible for CRC resistance to chemotherapy[41]. The inhibition of the Wnt/β-catenin pathway can restore the CRC sensitivity to 5-FU, thus exhibiting potential therapeutic potentials[42]. JAK/STAT signaling has been reported to regulate cell survival, proliferation, apoptosis, and differentiation, which is associated with CRC cells resistant to chemotherapy and immunotherapy[43-45]. TGF-β signaling also participates in the development of CRC resistance to chemotherapy through the upregulation of mesenchymal maker expression and inducement of MEK/ERK activation[46,47]. Targeted therapy against TGF-β thus presents promising value toward reducing the growth of chemo-resistant CRC cells and facilitating tumor control[48]. Last but not least, PI3K/AKT/mTOR pathway is well-accepted to be involved in tumorigenesis and development of CRC, which also contributes a lot to development of chemotherapy resistance[49]. Recent study identified miRNAs responsible for 5-FU resistance of CRC cell lines through regulating activity of PI3K/AKT pathway[50]. Moreover, PI3K/AKT/HIF-1α and AKT/GSK-3β/Snail signals also contribute to drug resistance via regulation of glycolysis and stem cell maintenance, respectively[51,52].
An increasing body of evidence has demonstrated crosstalk between tumor cells and the microenvironment, and the impact of the TME on chemotherapeutic efficacy suggests another mechanism for drug resistance. For instance, tumor-associated macrophages (TAMs) facilitate tumor growth, metastasis, and angiogenesis, while suppressing the anticancer immune response, regulating metabolism, and even influencing the microbiota[53]. Recently, multi-omics research utilized a large sample to reveal increased infiltration of neutrophils and monocytes in chemotherapy-resistant CRC tissues, which has been associated with poor outcomes[54]. Meanwhile, another research by Gupta et al[55] investigated metastatic CRC patients who received neoadjuvant chemotherapy. The results revealed that the TME was significantly altered after chemotherapy when neoadjuvant chemotherapy increased the levels of T cells, especially CD8+ T cells. Furthermore, bioinformatic studies focused on the prediction of sensitivity to chemotherapy and other therapies analyzing features of the TME and other pathological conditions have provided some promising results[56,57].
Epithelial-mesenchymal transition (EMT) also represents another form of interaction between tumor and microenvironment, especially during development of chemoresistance. Most tumor cells with the capacity of chemoresistance show a strong mesenchymal phenotype. EMT driven by miRNAs, oncogenes, protein kinases, and other factors facilitates to generation of drug resistance. Upregulated expression of DPYD, TYMS, ERCC1, and GSTP1 was associated with EMT, which eventually leads to 5-FU and oxaliplatin resistance of CRC cells[58,59]. DSTYK, a novel protein kinase, also contributes to chemoresistance in CRC cells by activating TGF-β induced EMT, and knockout of DSTYK hinders tumor growth in vivo[60]. Recently, proven by Zhu et al[61], activation of SOX2-β-catenin/Beclin1/autophagy signaling generated promoting effects on EMT, stemness and chemoresistance in CRC.
In addition to EMT, extracellular vesicles (EVs) are considered as a major pathway for cell-cell communication via transfer of their cargo, which ranges from proteins, lipids, miRNAs, and circRNAs. Due to the diversity of EVs cargo, it plays essential regulatory role during chemoresistance[62]. So far, compelling evidence indicated miRNAs transferred by EVs can be utilized as predictive marker of chemoresistance, and miR-21 was identified as the most common EV-associated miRNA relating to chemoresistance in CRC[63]. It was indicated that CRC exosomes are able to activate Wnt/β-catenin pathway through promoting the stabilization and nuclear translocation of β-catenin leading to 5-FU and oxaliplatin resistance[64]. CircRNA-122 delivered by EVs originated from chemoresistant CRC cells, played robust promoting effect on glycolysis which reduced drug susceptibility in chemosensitive cells[65]. In addition to harboring regulatory proteins, miRNAs, and circRNAs, drug efflux pumps (such as P-gp 1) and even cytotoxic drugs can also be transported by EVs to sequester drugs and negate drug effect on tumor cells[66-69]. Moreover, EVs generated from TME are also responsible for generation of chemoresistance of CRC. In a pre-clinical research, cancer-associated fibroblast (CAF)-derived EVs showed remarkable inhibitory effect on anti-cancer activity of oxaliplatin[70].
Radiotherapy resistance
Radiotherapy is considered another important adjuvant treatment to treat CRC, especially for advanced rectal cancer (as recommended by the NCCN guideline). However, resistance to radiotherapy is prevalent, which can be attributed to two main factors: tumor heterogeneity and the complexity of TME. Tumor heterogeneity is the most important reason for radiotherapy resistance, as supported by the fact that the equal effect of radiotherapy is not achieved in all tumor subpopulations. Among them, CSCs have been implicated as the most responsible factor for resistance to several therapies including radiotherapy[71-73]. Resistance to radiotherapy of CSCs can be categorized as intrinsic and acquired resistances, the latter of which is the adaptive response caused by radiotherapy itself. Owing to the distinct quiescent state and the plasticity of CSCs, they can survive stress, chemotherapy, and radiotherapy, which is referred to as intrinsic resistance[74]. Past studies have demonstrated that the activation of several signaling pathways contributes to CSC radio resistance, which includes Wnt/β-catenin, Notch signaling, TGF-β, Hedgehog, and PI3K/AKT/mTOR signaling. Wnt signaling increases the active levels of β-catenin post-radiation, leading to the development of resistance, which plays a role in the dedifferentiation of CSCs by regulating the SOX2 expression[75-78]. The Notch pathway can be activated via radiation and initiation of gene transcription, leading to an increased proportion of cells in the S phase[78]. The Hedgehog pathway is involved in the reprogramming of CAFs and is also responsible for the production of a supportive condition for the maintenance of CSCs stemness[79]. In addition to the alterations of the signaling pathways, tumor cells, especially CSCs, take adaptions of apoptosis, cell cycle, and EMT to survive radiation[74]. The cell-cycle progression can be blocked or slowed by irradiation that induces the redistribution of cells in the S phase, thereby contributing to radio resistance[80]. Researchers have found that the EMT triggered by irradiation may be related to the intravascular behavior of tumor cells forming the circulating tumor cells (CTCs) that are deemed responsible for the recurrence and metastasis[81,82].
Currently, a plethora of evidence indicates the significance of TME involved in radio resistance of solid tumors[74,83,84]. Concerns regarding the TME on radioresistance are based on the observation of COMMA-D cells, which are rarely tumorigenic and present with several features of normal mammary epithelial cells. In a mouse model, the implantation of unirradiated COMMA-D cells into the mammary fat pads of irradiated hosts eventually formed large tumors, indicating the pivotal role of the TME in the determination of the radiotherapy outcome[85]. Generally, the effects of radiotherapy on the TME can be categorized into 3 forms: Vasculature, stroma, and immune system[84]. Irradiation damages the vasculature within tumors by causing the dysfunction of endothelial cells. The main consequence of radiation is hypoxia induced by increased permeability and detachment of the endothelial cells from the basement membrane as well as apoptosis[86,87]. To date, hypoxia has been recognized as one of the most critical reasons for radio resistance of tumors, and several strategies have been devised to reverse this resistance by overcoming the hypoxic environment. Ritter et al[88] reported that exposure to acute and adaptation to chronic hypoxia alters the balance of Bcl-2 family proteins in favor of anti-apoptotic family members, thus attenuating the success of radiotherapy[88]. Moreover, exposure to hypoxia can activate oxygen-sensitive signaling cascades leading to metabolic adaptation and increasing cell death threshold, which accounts for a worse response to radiotherapy[89].
Regarding the impact on the stroma, radiotherapy induces significant activation of CAFs. The upregulation of integrin by CAFs participates in radio resistance through the interactions among cancer cells, stroma cells, and the environment[90,91]. Finally, the immune effect of radiotherapy has been well studied considering that induction of immunogenic cell death (ICD) after irradiation can, in turn, induce anticancer response through the release of neoantigens[92]. The resultant change in inflammation can be caused by the alterations in the cytokine signaling pathways in the TME under hypoxia-created irradiation, which recruits both immunostimulatory and immunosuppressive cells to locate the tumor site[93,94]. Moreover, the exhaustion of CD8+ T-cell induced by radiotherapy is another radio-resistant mechanism that is supported by the upregulation of PD-L1 on tumor cells after radiation therapy[95-97]. In such a case, the final immunomodulation of radiation therapy can be stimulatory or suppressive, which offers therapeutic challenges to confer the synergistic potential of radiation therapy combined with immunotherapy[84].
Immunotherapy resistance
Immunotherapy has long been recognized as an effective modality to treat multiple malignancies, albeit its efficacies on CRC remain confined to only a few subtypes. Generally, resistance to immunotherapy can be attributed to tumoral and microenvironmental factors. With regard to the tumor factors, it is well-acknowledged that CRCs with microsatellite stability is characterized by considerably low tumor mutation burden (TMB) and less immune infiltration when compared with CRCs exhibiting MSI. FDA-approved immunotherapy suggested certain outcomes in CRC patients with MSI metastatic disease[98,99]. In addition to MSI, two other pathways have been reported to be responsible for genomic instability, including chromosomal instability (CIN) and the CpG island methylator phenotype, whose identification can facilitate the stratification of immunotherapy-sensitive patients. In theory, the response to ICI is expected in all MSI tumors; however, a certain proportion of patients continues to experience progression due to several evading mechanisms in real clinical practice. Whether intrinsic or adaptive, the specific evading mechanism is not fully understood at present, although changes in some of the signaling pathways and gene expression have been reported[37,100,101].
The main mechanism concerns impaired antigen presentation or altered expression of HLA complex. Mutations of B2M [β2-microglobulin (B2M), or HLA class I heavy chain] in CRC have been reported to be significantly associated with the MSI phenotype but not MSS tumors[102,103]. Moreover, CRC patients with an MSI phenotype and harboring B2M mutations revealed resistance to anti-PD-1 mAb treatment[104]. B2M-mutant MSI tumors showed higher infiltration of PD-1+ T cells relative to B2M-wild-type MSI tumors, indicating the loss of HLA-I expression mediated by B2M mutation[105]. Moreover, the mutations of genes involved in the antigen presentation process, such as TAP1 and TAP2, have been related to the number of TILs in a tumor[106]. Other factors, including mutations in the HLA peptide-binding area, HLA class I transactivator NLR5, and RFX5, have also been cited as responsible for the altered function or expression of HLA that results in the development of immunotherapeutic resistance[101,106,107]. As for the signaling pathway, IFN-γ signaling plays an essential role in immunotherapy. The mutations of JAK1 and JAK2-the downstream kinases of IFN-γ signaling-are associated with an inherent resistant to anti-PD-1 mAb treatment of CRC[45]. Recently, the KRAS-IRF2 axis was illustrated to be responsible for the direct regulate of the CXCL3 expression, which bound to CXCR2 on the surface of myeloid-derived suppressive cells (MDSCs) and promotes the migration of MDSCs to TME, resulting in the development of immunotherapeutic resistance[108].
On the other hand, TME is significantly related to resistance to immunotherapy. Most importantly, the density, function, and site of immune cells’ infiltration in tumors are critical to the efficacy of the applied immunotherapy[109,110]. In such a situation, immunoscore is recommended for the evaluation of the immune contexture within a tumor. A score of 0-4 indicates a significant association with the prognosis of CRC patients[111]. Specifically, the expression of PD-L1 has been associated with a higher immunoscore[112,113]. Furthermore, researchers have suggested a novel CRC molecular subtyping system, and four consensus molecular subtypes (CMS) have fully illustrated CRC with distinct molecular and immune features[114]. According to the CMS subtyping, immunotherapeutic efficacy can be successfully predicted by different TMB and immunoscore, which is believed to be superior to those of conventional UICC-TNM staging[106,114-118]. Among the infiltrated immune cells, MDSCs and TAMs, which are referred to as immunosuppressive myeloid cells, are the major suppressive factors that downregulate the antitumor effect of CD8+ T cells. Recently, researchers revealed a novel mechanism responsible for the regulation of MDSCs function through prostaglandin E2 receptor 4 (EP4), and they developed a selective antagonist TP-16 as a potential target for the enhancement of immunotherapy for CRC[119]. Furthermore, the microbiota has also been implicated in the development of immunotherapeutic resistance of CRC[120]. Group 3 innate lymphoid cells (ILC3s) was recently reported to be associated with the alterations in microbiota in human along with the indications of dysfunctional adaptive immunity and immunotherapy resistance[121].
Additionally, many studies have linked tumor EMT phenotype with immunosuppression status through regulating PD-L1 expression, which lead to resistance to therapies. Evidence from a former study has revealed the link between EMT and CD8+ T cell immunosuppression. MiR-200, a cell-autonomous suppressor of EMT, regulates expression of PD-L1, but suppressed by EMT activator ZEB1[122]. Snail, a transcription factor of EMT, was shown to promote tumor growth in vivo and confer resistance against T cell-mediated lysis through the induction of autophagy[123,124].
Targeted therapy resistance
In addition to chemotherapy, radiotherapy, and immunotherapy, specific antibodies and small inhibitors therapy targeting cancer-associated receptors or signaling pathways represent another antitumor strategy toward control CRC. There are four main types of targeted therapy applicable in treating metastatic CRC: Monoclonal antibodies targeting EGFR, inhibitors of VEGF, small chemicals targeting intracellular kinases of several signaling pathways, and a recently approved small molecular BRAF inhibitor[125]. Among these, anti-EGFR therapy is widely used to manage KRAS wild-type CRC, including cetuximab and panizumab, the resistance to which will be mainly illustrated in this subsection. Reportedly, anti-EGFR therapy has been effective in 50% of CRC patients showing KRAS wild-type, but only 6% in those showing KRAS-mutant tumor[126,127]. In a clinical setting, patients usually develop resistance to anti-EGFR therapy after 3-12 mo of initiating the treatment[128,129]. Furthermore, it has been estimated that 80% of mCRC patients develop resistance to anti-EGFR regimens[130].
The mechanism of resistance to anti-EGFR therapy can be categorized into 2 types: Tumoral and environmental. Alterations of the EGFR ligands and EGFR significantly contribute to treatment resistance. The mutation of the EGFR kinase domain (V843 I) and the methylation of R198/R200 have been correlated with disease progression in patients receiving cetuximab[131]. Sequence changes in the extracellular domains of EGFR have also been implicated in conferring resistance to anti-EGFR treatment through the prevention of mAb binding[132-134]. The activation of compensatory feedback loop signaling, including RAS/RAF/MEK and PI3K/AKT/mTOR pathway, is also known to contribute to drug resistance[135]. Mutations, amplification, and the loss of genes, as well as aberrant phosphorylation of these pathways, are all important factors in the primary and secondary resistances to anti-EGFR therapy[136,137]. For instance, mutation of BRAF has been identified as the major marker of poor prognosis of mCRC after anti-EGFR treatment[138-140]. As a dominant downstream factor of EGFR signaling, mutations and abnormal activation of the PI3K/AKT/mTOR pathway is responsible for resistance to anti-EGFR[136,141,142]. Genomic stability is not only related to immunotherapy efficacy but also the development of anti-EGFR resistance. Moreover, it has been reported that hMLH1 deficiency generated resistance to cetuximab through the increased expression of ERBB2 and downstream PI3K/AKT signaling[143]. On the other hand, EGFR-targeted treatment has been reported to increase the levels of immune cells’ infiltration and PD-L1 expression, suggesting the potential of treatment combination with immunotherapy to combat the resistance of CRC[135].
CSCs are considered the predominant reason for treatment resistance. After targeted therapy, CSCs aberrantly activate the substitute receptor tyrosine kinase, leading to the development of resistance to anti-EGFR therapy[144]. Moreover, they activate anti-apoptotic signaling to generate resistance to bevacizumab, such as PP2A, p38MAPK, MAPKAPK2, and Hsp27[145,146]. During an antitumor treatment, cancer cells takeover metabolic remodeling to sustain survival, which contributes to the development of resistance to targeted therapy. It has been reported that elevated levels of glycolysis mediated by TRAP1 is related to resistance to anti-EGFR therapy and that fatty acid metabolism demonstrated certain anti-apoptotic effect during the cetuximab treatment[147,148]. Furthermore, autophagy is responsible for developing resistance to the anti-EGFR regiment[149,150].
In terms of the environmental factors, TME plasticity plays a significant role in modulating resistance to targeted therapy[149]. The dysfunction of immune cells, altered infiltration of CAFs, and angiogenesis all have an impact on the efficacy of anti-EGFR treatment[151]. Hepatocyte growth factor (HGF) secreted by CAFs binds MET receptor and activates MAPK and AKT signaling, thereby inducing resistance of CRC cells to cetuximab[144]. Moreover, Woolston et al[129] applied genomic and transcriptomic analyses to evaluate primary and secondary resistances of CRC during anti-EGFR treatment and revealed that NF1 and non-canonical RAS/RAF aberrations are associated with primary resistance. Importantly, stromal remodeling mediated by CAFs has been indicated as a non-genetic mechanism of cetuximab resistance[152]. In addition, treatment-induced senescence is believed to exert a pro-tumorigenic effect, leading to recurrence and progression, which can be attributed to the secretion of several factors and genetic mutations[153,154].
To compensate for the prevalent resistance to anti-EGFR regimens, the combined application of other targeted therapies has been proposed and tested in preclinical and clinical studies. Although several agent-targeted RAS mutations have been approved in the treatment of CRC[155-157], the resultant response is not sufficiently long for most patients, and the underlying mechanism included the upregulation of some compensatory signaling events such as the TME modulation and recruitment of CD11b+ Gr1+ myeloid cells[158]. Anti-IGF-1R treatment has been recognized as a targeted strategy in the treatment of mCRC, the resistance to which has also been encountered in clinical studies. The underlying mechanism can be attributed to the nuclear location of IGF-1R during the treatment, which leads to the development of resistance to anti-IGF-1R therapy as well as chemotherapy[159]. In a study of 47 human CRC cell lines, the authors observed a synergistic inhibition effect with the combination of MEK and PI3K inhibitors, although primary and secondary resistances were observed and the mutation of ERK2 was recommended as the driver of resistance to the mono- or combined regimens[160].
Since approved by FDA in 2004, Bevacizumab (Avastin) and other VEGF pathway inhibitors have been utilized for cancer therapy, which improved survival in most cancer patients but some still have limited or no benefit from them. The primary and acquired resistance can be attributed mainly to activation of alternative angiogenesis other than VEGF pathway, including angiopoietin (Ang2), bombina variegate (Bv8), FGF, etc[161,162]. Additionally, other factors also play important role in development of anti-VEGF therapy resistance. Inflammation was tested to affect responsiveness to anti-VEGF in a mouse model of chemical induced colitis, which revealed Bv8/PROK2 expressed by tumor-infiltrating neutrophils related to anti-VEGF resistance[163]. Specifically, patients with CD177+ neutrophil infiltration in tumor tissues had poor survival than those with CD177- infiltrates[164]. Besides, HOXB9 and extracellular matrix (ECM) remodeling induced by anti-VEGF were responsible for development of anti-VEGF resistance[165,166]. Park et al[167] found that nearly all of the cetuximab-resistant colorectal cancer cells showed a higher EMT signature, showing increased EMT markers such as SNAI2.
Metastasis
Metastatic events are the major cause of CRC patients’ death, with the liver being the most common site of metastasis[168-170]. The lung, distant lymphatic nodes, and peritoneum are the secondary metastatic sites of CRC[168]. In the 1880s, the classic concept of “seed (tumor cells) and soil (specific organs) was proposed by Paget, which illustrated the basic metastatic mechanism for tumors[171]. To date, several excellent reviews have detailed the specific intravasation and extravasation procedures of CRC cells during metastasis[172-176]. For instance, liver metastasis is recognized as multiple factors- and processes-involved complex biological procedures. First, a certain subtype of CRC cells is endowed with the invasion capability, and they migrate to the ECM through the EMT, followed by infiltration into the surrounding tissues, after which they enter and survive in circulation, eventually colonizing and forming a secondary tumor in distant organs via extravasation[172]. Moreover, a metastatic mouse model explored by Enquist et al[177] suggested that the direct spread of CRC cells through the blood to the liver is a possible dissemination route without any prior lymph-node involvement; this information has the potential to facilitate future drug development targeting metastasis[177,178].
As far as the intrinsic factors are concerned, several molecular mechanisms have been deemed responsible for CRC metastasis, including non-coding RNAs (ncRNAs), Notch, TGF-β, and exosomes[172]. Among these, TGF-β is widely accepted as the most important signaling pathway related to metastasis. It was recently reported that an increased level of TGF-β in the TME promoted T-cell exclusion and blocked the acquisition of the TH1-effector phenotype, while the blockage of TGF-β signaling rendered CRC susceptible to immunotherapy[179]. To induce the EMT process, TGF-β downregulated the expression of E-cadherin and upregulated the Vimentin expression in CRCs in order to promote invasion and migration[180]. Consequently, TGF-β signaling may serve as a vulnerable factor of CRC metastasis that can be exploited for targeted therapy in a clinical setting.
In addition to the EMT process mentioned earlier, recent studies have indicated a cell death process-dependent pathway for metastasis[181]. Among the several reversible cell death forms (such as apoptosis, necroptosis, ferroptosis, pyroptosis, and NETosis), the Blebbishield emergency program is considered a prototype for cell death-driven metastatic pathway[176]. The Blebbishield metastatic-witch pathway is characterized by the formation of apoptotic blebbing that is reconstructed into blebbishields and, subsequently, a spheroid state. This fusion structure may release or generate single cancer cells with the ability of migration, genomic instability, immune evasion, and increased metastasis[176,182-185]. On the other hand, it is believed that tumor cells must overcome multiple environmental obstacles during metastasis, and metabolic rewiring is widely adapted to achieve successful colonization in distant organs[186]. The adaption of pyruvate, lactate, glutamine, and fatty acids metabolism all contribute a several invasion and migration stages, the circulating stage, and the final colonization step, which represents a potential target for metastasis prevention and treatment[186-189].
Beyond the intrinsic factors of altered signaling and metabolic adaption, extrinsic conditions also participate in the regulation of CRC metastasis. Recently, neutrophil extracellular traps (NETs) formed by decondensed chromatin filaments of neutrophils were implicated in cancer metastasis[174,190]. Clinically, the increased accumulation of neutrophils has been revealed in pre-metastatic organs[191,192]. Moreover, immunostaining of the CRC tissues demonstrated the existence of NETs in primary and related metastatic lymph nodes of CRC[193]. Through in vivo and in vitro assays, NETs were found distributed at the primary site and over the tumor boundary of CRC, whose levels were increased in CRC patients[193-196]. The relevance between NETs and CRC progression and prognosis was illustrated with the assistance of a mouse model and an observational study[197]. Functionally, NETs promote proliferation and the secondary metastasis of CRC, as well as prepare for the adhesion of CTC to the liver or the lung epithelial cells[198-202]. It was also reported that the disruption of NET formation by DNase facilitates the control of CRC metastasis[194,196,199,203,204]. As a pivotal component of the TME, immune cells were found to be involved in CRC metastasis. TAMs have been reported to induce the EMT process for the promotion of the metastatic capability of CRC and CTC-mediated metastasis, which subsequently lead to the production of CCL2 for enhancing macrophage recruitment[205].
APPLICATION OF NANOTECHNOLOGY IN CONQUERING THERAPEUTIC RESISTANCE AND METASTASIS
As mentioned earlier, 2 main reasons result in treatment resistance and metastasis of CRC, namely tumor and environmental factors. Despite the advancements in cancer treatment and the development and design of several drugs or methods to target metastatic sites or reverse drug resistance, only a few such approaches have achieved satisfactory clinical outcomes. The rapid advancements in nanotechnology have provided new methods and options for the treatment of cancer with unique advantages, such as an excellent carrying capacity, better permeability, and the realization of multidisciplinary therapy[206]. In terms of nano delivery methods, compelling reviews have fully illustrated the diversity of nanomaterials currently utilized for cancer therapy, including organic materials, inorganic materials, polymers, and exosomes, etc. Exosomes, particularly, have been explored as a potential candidate in therapeutics delivery owing to their intrinsic targeting property, endogenous functionality, and ability to cooperate with a host defense mechanism. So far, exosome-based nanotechnology has achieved marked technological advances and exosome-based nanotheranostic platforms have thus been bloomed[207]. It has been demonstrated that exosome delivery led to higher drug accumulation in target cancer cells and improve molecule stability as well as blood circulation time, thus improving the efficiency of small molecule drugs[208]. Besides, exosomes secreted by different cell types contains a diversity of proteins which facilitates better recognition of targeted cells and reduce nonspecific distribution[209,210]. In general, nanotechnology-based strategies have been developed to restore drug sensitivity and enhance the anti-tumor efficacy. The detailed advancement in accordance with the mechanism of therapeutic resistance and metastasis, will be discussed in detail in the following section.
Nanotechnology and surgery
Until date, surgery remains the preferred scheme of CRC and the only curative option for malignant bowel obstruction in some cases. As surgical procedures facilitate thorough and timely removal of the primary cancer site, the possibility of disease cure is ensured in most cases. However, 2 major challenges remain, including precise identification of micro-metastatic lesions during the surgery and the generation of a suppressive immune microenvironment after the surgery. Taking advantage of fluorescence imaging technology, researchers have developed novel intraoperative imaging methods that can specifically detect the tumor edge and micro-metastatic sites. For instance, Li et al[211] designed a fluorescent probe activated by a CD13/aminopeptidase N (APN), YH-APN, which demonstrated superior tumor-to-normal (T/N) tissue ratios under different conditions. Moreover, owing to the superior ability to distinguish metastatic lesions, even those as small as 1 mm in diameter, image-guided resection, and imaging of metastatic cancer were successfully achieved by spraying YH-APN. Recently, Wang et al[212] also used an in situ spraying method that enabled precise distinguishing of the tumor edge under the guidance of fluorescent probes. During surgery, the use of real-time fluorescent imaging to detect β-glucuronidase (GLU) activity was achieved by DCDNO2, and the outlines of the tumor were visualized, which guided precise resection of liver cancer.
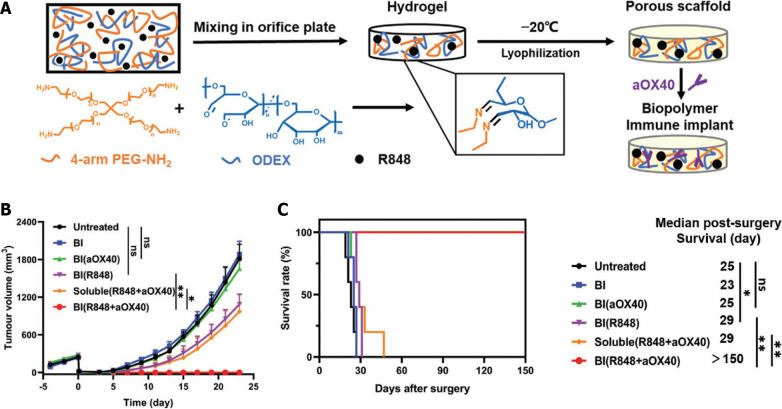
Regarding the suppressive immune microenvironment, Ji et al[20] designed a biopolymer nanogel implant that can be placed in the resection cavity of CRC (Figure 2). Owing to its good tissue adhesion ability and biodegradability, the implant remained in the surgical site and the loaded drugs, including antibody and resiquimod (R848), were released gradually. In the implant group, the residual tumors were eradicated after surgery, which helped inhibit distal tumor metastasis and induced immune-memory effects resisting the re-challenge experiment. Recently, Si et al[21] further improved a nanogel implant to an in-situ-sprayed gel delivering anti-OX40 antibody, which demonstrated the promising therapeutic potential of CRC. This improved nanogel modulated the suppressive immune microenvironment by inhibiting the activity of cyclo-oxygenase-2 (COX-2); as a result, the loaded aOX40 was gradually released for over 20 days, serving as an immune agonistic antibody. The treated mice displayed resistance to the re-challenge with M38 cells and a remarkable inhibition of the growth of abdominal metastatic tumors[21].
Figure 2.
Intraoperative implant of biopolymer exhibited therapeutic effect on colorectal cancer growth. A: Schematic illustration of the implant preparation process; B: Immunoimplant inhibited tumor growth post-surgery; C: Immunoimplant showed survival benefit after tumor re-challenge. Citation: Ji G, Zhang Y, Si X, Yao H, Ma S, Xu Y, Zhao J, Ma C, He C, Tang Z, Fang X, Song W, Chen X. Biopolymer Immune Implants’ Sequential Activation of Innate and Adaptive Immunity for Colorectal Cancer Postoperative Immunotherapy. Adv Mater 2021; 33: e2004559[20]. Copyright ©The Author(s) 2021. Published by Wiley-VCH GmbH Publications. The authors have obtained the permission for figure using from the Wiley-VCH GmbH Publications (Supplementary material).
Nanotechnology and chemotherapy
The therapeutic strategy of nanomaterials-derived drug delivery system toward overcoming chemotherapy resistance can be generally summarized into 4 aspects: (1) Increasing the drug concentration, accessibility, and permeability; (2) targeting the key genes of drug metabolism; (3) targeting the underlying efflux mechanism of the drug; and (4) the delivery of phytochemicals as an alternative.
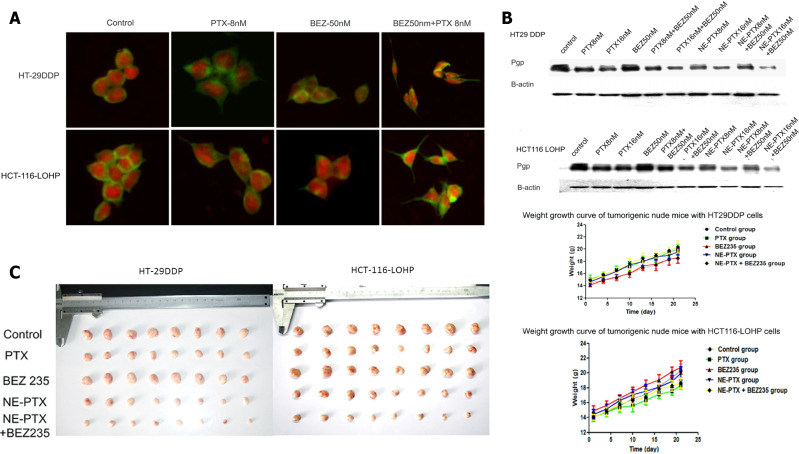
The commonly applied strategy for overcoming drug resistance is to increase the local drug concentration. Improved cytotoxicity to human CRC cell lines was achieved through the direct delivery of DOX and 5-FU via oleic acid, which reduced the IC50 values relative to that with the free-drug forms[206,213]. The codelivery of chemotherapy and targeted therapy by nanomaterials also demonstrated an improved synergistic effect on the CRC cells. Recently, nanoemulsion loaded with paclitaxel (PTX) and BEZ235-a PI3K/AKT/mTOR pathway inhibitor-displayed a greater antitumor effect with remarkably lower IC50 when compared with free PTX. The combined therapy with nanoemulsion inhibited the growth of drug-resistant CRC cells either in culture or in vivo (Figure 3)[214]. Furthermore, theranostics mediated by magnetic resonance imaging (MRI) was applied to combat drug resistance through precise diagnosis and targeted therapy[215]. The application of the pH-responsive polymer PEALCa nanomicelles loaded with PTX and SPIO avoided the uptake by RES, entered human CRC cells, reached the lysosomes, and released PTX in vitro. The drug uptake efficiency could be visualized by MRI, which exhibited a relatively more efficient antitumor effect[216]. Further attempts took advantage of the imaging characteristics of materials to deliver drugs, realize real-time imaging of the drug uptake, as well as to control tumors simultaneously[217-219]. The decreased permeability of a drug is another reason for the development of resistance to it. For instance, Bergers et al[186] designed a nanogel loading 5-FU nucleoside floxuridine or other chemical agents, like gemcitabine. Their conjugated nanogel-chemotherapy system exhibited an improved anticancer effect on drug-resistant Caco-2 cells, with enhanced permeability across the gastrointestinal barrier.
Figure 3.
Nanoemulsioned Paclitaxel and BEZ235 reduced multidrug resistance of colorectal cancer. A: Morphological changes of cells treated with nanoemulsioned Paclitaxel and BEZ235; B: Downregulated expression of P-gp after NE-PTX and BEZ235 treatment; C: Synergistic inhibition of CRC growth in vivo. Citation: Hu Y, Zhang K, Zhu X, Zheng X, Wang C, Niu X, Jiang T, Ji X, Zhao W, Pang L, Qi Y, Li F, Li L, Xu Z, Gu W, Zou H. Synergistic Inhibition of Drug-Resistant Colon Cancer Growth with PI3K/mTOR Dual Inhibitor BEZ235 and Nano-Emulsioned Paclitaxel via Reducing Multidrug Resistance and Promoting Apoptosis. Int J Nanomedicine 2021; 16: 2173-2186[214]. Copyright ©The Author(s) 2021. Published by Dove Medical Press Ltd. The authors have obtained the permission for figure using from the Dove Medical Press Ltd. (Supplementary material).
Targeting mutations or the altered expression of genes involved in drug metabolism also serves as a promising option to resolve the issue of drug resistance. The upregulated expression of DPD (DPYD) has been implicated in the development of 5-FU resistance, as it catalyzes 5-FU into an inactive metabolite[220,221]. With the intension to reverse the resistance to 5-FU of SW480 cells, Chen et al[189] designed hollow mesoporous silica nanoparticles (HMSN) to deliver 5-FU. When grafted with EGF targeting to EGFR-overexpressed SW480/ADR cells, elevated intracellular 5-FU was recorded, which induced apoptosis and cell-cycle arrest, along with the downregulation of DPYD. In addition, restorage of sensitivity to 5-FU was achieved with the use of multi-walled carbon nanotubes and several other nano-based delivery systems with the capability to boost the antitumor efficacy of chemotherapeutics[222,223].
Improvement in the biodistribution and pharmacokinetics of cytotoxic drugs is possible through the adoption of an environmentally responsive nanomaterial-derived delivery system. As reported by Udofot et al[224], pH-sensitive liposomal nanoparticles entrapping 5-FU modified with an anti-EGFR antibody (pHLNps-5-FU) exhibited prolonged plasma circulation, while accumulated exposure of pHLNps-5-FU in a tumor site exhibited enhanced anticancer effect relative to that with a free drug[224]. Au nanoparticles (AuNPs) are used to increase the 5-FU efficacy and decrease the related side effects; moreover, AuNPs-based nanoformulation has been reported to enhance the cytotoxicity of 5-FU to CRC cells[225]. Targeting the efflux mechanism to increase the intracellular accumulation of drugs serves as another effective approach to restoring drug sensitivity. For example, NPs designed with a PLGA core and an HA polymer shell loading 5-FU and L3-encoding cDNA (plasmid, pL3) were reported to sensitize CRC cells to 5-FU, as pL3 protein interfered with drug efflux via the P-gp regulation[226].
In addition to the abovementioned efforts, alternative therapies have achieved considerable outcomes with the use of naturally available phytochemicals, including curcumin, genistein, lycopene, and resveratrol, among others[227]. Thanks to nanotechnology, novel nanoformulations delivering phytochemicals have realized more efficiency and reduced side effects, which compensate for poor absorption, low solubility, and rapid metabolization of these natural drugs. Liposomes have been applied to enhance the uptake rate of curcumin, and its IC50 is lower than that of oxaliplatin, which can reduce the expression of angiogenic factors, increase apoptosis, inhibit angiogenesis, and prevent CRC progression[228]. The simultaneous encapsulation of curcumin and DOX in liposomes have been reported to increase drug accumulation at the CRC sites, exhibiting a higher inhibitory effect compared to that with free DOX[229]. Similarly, the establishment of an amphiphilic block copolymer pluronic/polycaprolactone to encapsulate curcumin demonstrated a better targeting effect on the tumor sites as well as restored the sensitivity of tumor cells to drugs[230]. Considering its abilities of chemosensitization, antioxidation, and anti-inflammation, resveratrol was loaded into gold nanoparticles (GNPs) after tagging with radionuclide technitium 99m; it was rapidly taken up by CRC cells, displaying obvious cytotoxicity to tumor cells and excluding normal colon cells[231,232]. In support, other past studies have confirmed that the combination of resveratrol with 5-FU increases the sensitivity of CRC cells to 5-FU[233-236].
Hui et al[237] loaded oxaliplatin into exosomes encapsulating PGM5 antisense RNA 1 by electroporation. The results suggested a significant inhibition of both CRC cell proliferation in vitro and tumor growth in vivo[237]. Tran et al[238] encapsulated aspirin into exosomes derived from colorectal cancer cells and strongly enhanced cytotoxicity of aspirin to cancer cells. Interestingly, this cytotoxic effect was more pronounced to parental cells of the exosomes, reminiscent of homing effect.
Nanotechnology and radiotherapy
Radiotherapeutic resistance is developed through multiple mechanisms, as illustrated in Section 2. Accordingly, strategies for treating radiotherapy resistance can be categorized into 2 aspects: Investigation of newer biomarkers or signatures to predict radiotherapy sensitivity and the modification of the TME, such as hypoxia. Due to the intrinsic resistance to radiotherapy, it is necessary to establish methods for the detection and identification of sensitive cases before initiating treatment. Recent studies have been able to investigate the genetic signatures and normalize clonogenic assays to predict the radiotherapy response in a more precise and effective manner[239,240]. On the other hand, the modulation of the TME by nanotechnology has demonstrated an improved radiotherapeutic efficacy owing to 3 advantages: An improvement of hypoxia by the loaded cargos, the radiosensitization capacity of some nanomaterials, and the combined application of multiple therapies by using a nanoplatform.
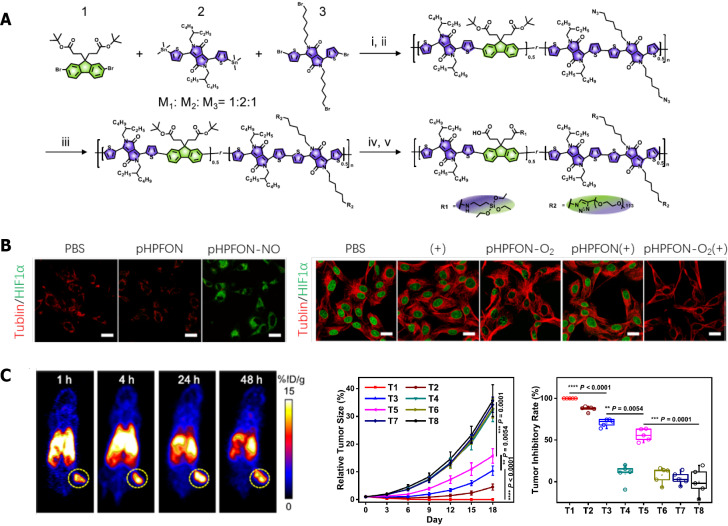
Hypoxia is believed to be one of the most prevailing mechanisms for the development of radioresistance. Methods suggested to restore the tumor oxygen supply before or during radiotherapy include erythrocyte transfusion, erythropoietin administration, and hyperbaric oxygen (HBO) treatment, among others. To date, compelling evidence has demonstrated that hypoxia can be successfully overcome with the use of relevant nanomaterials, as also summarized by several excellent reviews[241-243]. For instance, some simulated oxygen- or water-soluble components can be employed to improve the effect of radiotherapy. In one study, the bioreactive albumin MnO2 nanoparticles synthesized by nanotechnology produced more oxygen in the hypoxic area, which was helpful to improve the radiotherapy effect[244]. Other strategies adopted so far include the delivery of exogeneous O2 to the tumor site, in situ generations of O2 and reduced consumption of O2 in a tumor. Moreover, Tang et al[245] developed a nanoeconomizer to overcome radio resistance by regulating the hypoxic environment within tumors. This system functions in two ways: First, it releases NO in the acidic microenvironment to protect endogenous oxygen supply and reduce oxygen consumption; second, the photothermal effect facilitates the clearing of the residual tumor lesions that are resistant to radiotherapy (Figure 4).
Figure 4.
Organosilica-based O2 nanoeconomizer improved radiotherapy efficacy. A: Design and synthesis of nanoeconomizer; B: pHPFON-NO reduced oxygen consumption (left), and broadened oxygenation (right); C: Multi-modal imaging by pHPFON-NO/O2, and inhibited tumor growth after pHPFON-NO/O2 administration. Citation: Tang W, Yang Z, He L, Deng L, Fathi P, Zhu S, Li L, Shen B, Wang Z, Jacobson O, Song J, Zou J, Hu P, Wang M, Mu J, Cheng Y, Ma Y, Tang L, Fan W, Chen X. A hybrid semiconducting organosilica-based O(2) nanoeconomizer for on-demand synergistic photothermally boosted radiotherapy. Nat Commun 2021; 12: 523[245]. Copyright ©The Author(s) 2021. Published by Springer Nature Ltd. The authors have obtained the permission for figure using from the Springer Nature Ltd. (Supplementary material).
Currently, nanoparticles with high atomic numbers, including metal-based NPs, have drawn much interest as radiosensitizers[246-248]. The level of GNPs taken up by CAFs in the TME is higher than that of tumor cells and normal fibroblasts. Therefore, during radiotherapy, GNPs can increase the local radiation dose and reduce the toxic effect on normal tissues, which improves the overall radiotherapy effect. Bromma et al[249] compared GNPs uptake by CAFs and DNA damage foci in CAFs to that in normal fibroblasts and HeLa cells and found that GNPs act as an effective tool in cancer radiotherapy to improve the overall efficiency. GNPs are commonly used as a radiosensitizer in in vitro experiments, albeit only a few have been used in clinical experiments due to their redox reactivity. Moreover, Guerra et al[18] designed an intercomparison of the respective sensitization capacities of GNP and iron oxide nanoparticles in human glioblastoma cells; they found different sensitization mechanisms, suggesting metal-based NPs as effective alternations to eliminate radioresistance.
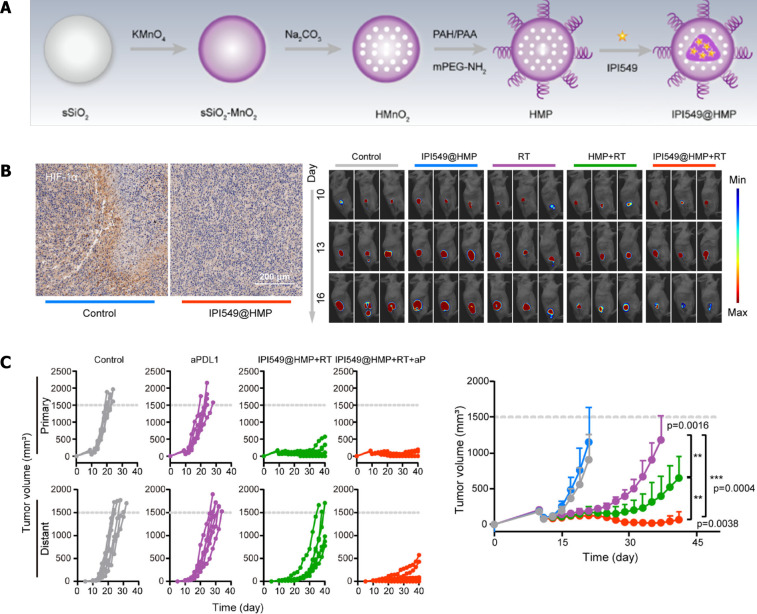
Finally, through functionalization, the nanomaterials-derived system can be developed to realize disciplinary treatment simultaneously. The combined utilization of radiotherapy and immunotherapy exhibited great synergistic potential in the treatment of cancers. To overcome hypoxia and an immune suppressive microenvironment, Li et al[19] constructed a nanoprobe based on quantum dots that emitted in the near-infrared IIb window. By aggregation of nanoprobes in a tumor site, precision radiotherapy was realized with only a few side effects, improved ICD-augmented activation of dendritic cells, and induced robust T cell-mediated antitumor effect. Recently, Guan et al[16] engineered a nanoplatform combining radiotherapy and immunotherapy and discovered the potential to remodel the suppressive microenvironment after surgery. They constructed nanomedicine IPI549@HMP-targeted myeloid cells and catalyzed endogenous H2O2 to O2, thereby reversing hypoxia-related radioresistance, which increased susceptibility to anti-PD-L1 therapy and eventually resisted tumor rechallenge.
Wang et al[250] developed exosomes from γδ-T cells and found that exosomes from γδ-T cells can specifically target the radioresistant CD44+/high CSCs in nasopharyngeal carcinomas. Moreover, exosomes combined with radiotherapy had a higher therapeutic potency than radiotherapy monotherapy in vitro and in vivo. Wan et.al found that microRNA-34c-5p (miR-34c) could inhibit malignant behaviors in nasopharyngeal carcinomas. Exosomes overexpressing miR-34c significantly increased radiation-induced apoptosis in nasopharyngeal carcinomas[251].
Nanotechnology and gene therapy
Gene therapy represents a novel tool for conquering therapeutic resistance of CRC in a specific and targeted method, considering that genetic alterations are generally responsible for treatment failure. Strategies involving gene therapy include gene replacement, gene addition, gene expression modifications at the RNA level, and gene editing at the DNA level[252,253]. The combination of molecular biological technologies and nanomaterials has significantly promoted the development of gene therapy for CRC. Pigment epithelium-derived factor (PEDF) protein, a well-accepted tumor suppressor, has been reported to inhibit tumor proliferation and progression through its anti-angiogenesis and pro-apoptotic effects. Recently, Bao et al[254] designed a liposome-loading PEDF-DNA decorated with an iRGD peptide and demonstrated the reduced metastatic capability of CRC cells and the induction of apoptosis in vitro, as well as the inhibition of lung metastasis and prolonged survival of the animal model.
In addition to delivering DNAs encoding functional proteins, gene silencing therapy is commonly applied in CRC treatment. The methods adopted for gene silencing usually comprise the delivery of antisense oligonucleotide, miRNA, and siRNA, among which siRNA delivery is believed to offer more advantages[255-259]. Moreover, CRISPR/Cas9 technology has achieved a better targeting effect relative to siRNA therapy[260,261]. Recently, Zhao et al[262] constructed a cationic liposome-based nanoparticle for purpose of delivering miR-139-5p, decorated with epithelial cell adhesion molecule aptamer on the surface for the targeted treatment of CRC. With the use of cell lines and animal models, the constructed nanoparticle exhibited a satisfactory uptake and targeting ability, while demonstrating a tumor-growth suppressive effect.
On the other hand, siRNA delivery has achieved considerable outcomes through silencing of the key genes related to metastasis and MDR in order to reverse drug resistance of CRC cells[263,264]. To date, several nanocarriers have been developed to deliver siRNAs, which include liposome, PLGA, and RNA nanoparticles. Liposome-derived system is the most common platform for delivering siRNAs, which can protect siRNAs from degradation and kidney clearance while offering the advantages of convenient manufacturing and biodegradability[263]. However, the utilization of liposomes continues to be hindered by issues related to toxicity and release, and PLGA polymers were recently proposed as the preferred agents to realize prolonged release circumventing these obstacles while showcasing better biodegradability and lower toxicity[264-266]. Thanks to rapid development, nucleic acid nanotechnology has gained much attention with its distinct features. Particularly, RNA nanoparticles are widely being used to deliver multiple drugs, thanks to their low toxicity, negative potential, programmable features, and the ability to self-assemble and multi-valent capacity[267]. Rychahou et al[268] developed a multifunctional RNA nanoparticle targeting FA and achieved an excellent target effect to CRC metastatic cells mediated by FA-receptor mediated endocytosis, which demonstrated no accumulation in the normal tissues of the targeting organs such as the liver, lymph nodes, and lung. Moreover, the combination of siRNA delivery with RNA nanoparticles was recently applied in the treatment of lung cancer, breast cancer, and prostate cancer, which effectively inhibited the Survivin expression and slowed the tumor growth in vivo[269].
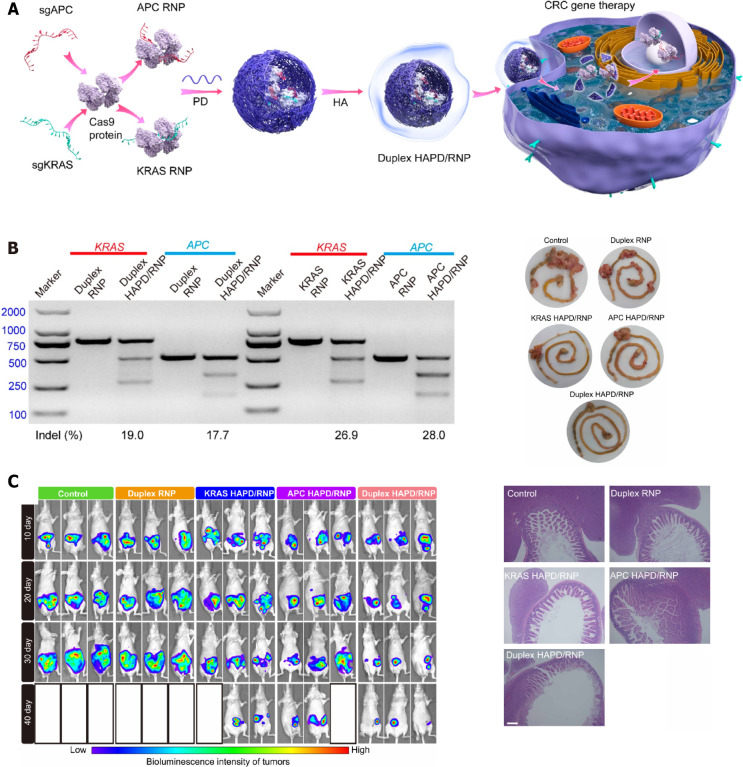
Taking advantage of the abovementioned nanocarriers, siRNA therapy can target various genes or be combined with other treatments. Antigen-presentation cells (APCs) and KRAS are well-known oncogenes responsible for CRC development. Recently, Wan et al[270] designed a nanomedicine based on the CRISPR/Cas9 technology, which simultaneously targeted APC and KRAS mutation CRCs. A duplex CRISPR/Cas9 ribonucleoprotein was designed to deliver APC and KRAS sgRNA encapsulated in a phenylboronic dendrimer decorated with hyaluronic acid (HAPD). The resultant nano-complex was administered systematically to Balb/c nude mice, which displayed a synergistic targeting effect on APC and KRAS mutations along with a remarkable reduction in tumor growth. Moreover, the therapeutic capacity for metastasis was further illustrated by the establishment of the orthotopic CRC mouse model and the liver and lung metastasis models, whose survival rates were significantly prolonged (Figure 5)[270]. Intergrin-β1 is one of the key factors involved in CRC metastasis, which is upregulated in CRC cells resistant to regorafenib[271]. The delivery of siRNA-targeting intergrin-β1 to regorafenib resistant CRC cells via regorafenib/dimethyldioctadecylammonium bromide (DDAB)-methoxy poly (ethylene glycol) (mPEG)-poly-ε-caprolactone hybrid nanoparticles (HNPs) was found to downregulate the intergrin-β1 expression and induce apoptosis, thereby successfully enhancing the overall sensitivity to regorafenib[271]. P-gp is responsible for drug pump leading to decreased drug concentrations in the cells, which has been proposed as another target of siRNA therapy. The delivery of P-gp siRNA with lipofectamine achieved an efficient control of CRC cells[272]. In addition, the delivery of siRNA-silencing TGF family loaded in tripolyphosphate-decorated chitosan nanoparticles inhibited the formation of liver metastasis of CRCs in vivo[273]. The co-delivery of multiple siRNAs or combination with other treatments, such as chemotherapy, can thus be considered as an alternative strategy for the improvement of CRC control[274].
Figure 5.
Duplex CRISPR/Cas9 ribonucleoprotein nanomedicine disrupted antigen-presentation cells and KRAS expression to treat colorectal cancer. A: Schematic illustration of preparation of nanoformulations; B: Disruption of antigen-presentation cells and KRAS expression after duplex HAPD/RNP treatment; C: In vivo luciferase expression of orthotopic CRC (left), and representative intestine images and H&E staining of intestine sections after nanomedicine treatment. Citation: Wan T, Pan Q, Liu C, Guo J, Li B, Yan X, Cheng Y, Ping Y. A Duplex CRISPR-Cas9 Ribonucleoprotein Nanomedicine for Colorectal Cancer Gene Therapy. Nano Lett 2021; 21: 9761-9771[270]. Copyright ©The Author(s) 2021. Reprinted permission from ACS Publications. The authors have obtained the permission for figure using from the ACS Publications (Supplementary material).
Li et al[275] suggested that miR-3940–5p is significantly downregulated in CRC. They loaded miR-3940–5p into exosomes derived from MSCs and transfected them into CRC cells. The results showed significant suppression of EMT and invasion in vitro, and inhibition of tumor growth and metastasis in vivo. Liu and coworkers evaluated the effect of MSC-derived exosomes transfected with miR-15a on CRC cells. The results indicated the suppression of CRC cell proliferation and tumorigenesis in vitro and in vivo[276].
Nanotechnology and immuno- and targeted therapies
Currently, it has been well-accepted that nanotechnology offers distinct advantages in improving the efficacy of cancer immunotherapy, which is widely used as the platform that enables the combined utility of immunotherapy with other treatments, along with the reprogramming of the tumor immunosuppressive microenvironment.
Firstly, it has long been established that targeted and immunotherapy can be combined with other anticancer treatments to achieve synergistic effects[6,277-279]. The introduction of nanotechnology to cancer immunotherapy, especially for CRC, is based on the hope of reversing the immunosuppressive microenvironment, inducting a robust immune response and long-term immune memory effect, as well as transforming non-responsive T cells into responsive T cells in the TME for MSS or pMMR CRC[280]. Ni et al[281], for instance, constructed a bi-adjuvant nanovaccine (banNV) that delivers the tumor antigen Adpgk, TLR7 agonist R848, and TLR9 agonist ODNs. The combined administration of banNV with anti-PD-1 antibody was found to suppress tumor growth in an orthotopic MC38 mouse model, prolonging the survival rate to 70% on day 48 of treatment. Furthermore, the use of nanomedicine co-delivering photothermal or photodynamic agents with immunotherapy treatment has bene reported to achieve a certain improvement in CRC control. Luo et al[282] took advantage of hollow gold nanoshell encapsulating anti-PD-1 peptide into biodegradable poly (D, L-lactic-co-glycolide) nanoparticles (AA@PN), as it overcomes the shortcomings of peptides that have a short half-life, poor stability, and off-target effects. On irradiation by a NIR laser, AA@PN generated photothermal ablation of tumors. In another study, through PD-1 blocking and photothermal ablation via NIR laser irradiation, perdurable and enhanced immune response was recorded in the CT26 mouse model that also prolonged the survival rate[282].
He et al[283] realized the potential of the combined application of chemotherapy, photodynamic therapy, and ICI therapy through the construction of a nanoscale coordination polymer core-shell nanoparticles-loading oxaliplatin in the core and photosensitizer in the shell. In CRC mouse models, the combined administration of nanoparticles and anti-PD-L1 antibody was found to promote the release of pro-inflammation cytokines and synergistically activate DC and CD8+ T cells, which significantly controlled the local and distant tumor growth, with the primary tumors reducing in size by 2.9% in the PBS-treated group[283]. In addition, nanotechnology offers an optimal platform for radiotherapy and ICI therapy. A nanoscale metal-organic framework was established to enable radiotherapy and checkpoint blockade immunotherapy (indoleamine 2,3-dioxygenase, IDO). The abscopal responses were generated after irradiation, leading to an enhanced immune response in both CRC and breast cancer mouse models[284]. Recently, Guan et al[16] developed a novel therapeutic strategy targeting the suppressive microenvironment post-surgery by using the nanomedicine IPI549@HMP, which was composed of a PEGylated HMnO2 (HMP) loaded with PI3-kinase γ (PI3kγ) inhibitor (IPI549). PI3Kγ, which is highly expressed on MDSCs, serves as the target molecule for the specific regulatory effect of IPI549@HMP on myeloid cells. Moreover, this nanomedicine catalyzed H2O2 into O2 by remodeling the hypoxia environment after radiation. In this case, the combined use of IPI549@HMP with anti-PD-L1 antibody exhibited a synergistic effect on the elimination of CT26 cells and the suppression of distant metastasis, which significantly prolonged the survival of IPI549@HMP-treated mice up to 100 d[16] (Figure 6).
Figure 6.
Nanomedicine targeted myeloid-derived suppressive cells exhibited synergistic inhibitory effect on colorectal cancer growth with radiotherapy and PD-L1 blockade. A: Schematic illustration of synthesis of HMP nanoparticles and drug loading; B: IPI549@HMP treatment improved hypoxia environment (left), and augmented radiotherapy efficacy; C: Kaplan-Meier survival curves and tumor growth curves of each groups. Citation: Guan X, Sun L, Shen Y, Jin F, Bo X, Zhu C, Han X, Li X, Chen Y, Xu H, Yue W. Nanoparticle-enhanced radiotherapy synergizes with PD-L1 blockade to limit post-surgical cancer recurrence and metastasis. Nat Commun 2022; 13: 2834[16]. Copyright ©The Author(s) 2022. Reprinted permission from Springer Nature Publications. The authors have obtained the permission for figure using from the Springer Nature Publications (Supplementary material).
On the other hand, nanotechnology also contributes to the modulation of the immunosuppressive microenvironment, as illustrated by several excellent reviews[285-287]. Targeting APCs augments the antitumor effect by inducing a more extensive T-cell response. Zhu et al[288] designed self-assembled interwining DNA-RAN nanocapsules (iDR-NCs) that delivered CpG-coding DNA and STAT3-shRNA. The nanocapsules activated APCs by TLR9 signaling and relieved the inhibition of the CpG immunostimulatory effect by silencing STAT3. In an MC38 mouse model, the nanocapsules generated durable T cell responses, demonstrating potent synergistic immunostimulation effect[288]. Importantly, high levels of intratumoral lipopolysaccharide (LPS) are related to a poor response to anti-PD-L1 antibody treatment. Consequently, Song et al[289] used polymyxin B (PmB) to trap LPS inside the tumor. In combination with the TLR4 agonist TAK-242, a remodeled TME was achieved that allowed enhanced infiltration of the T cells in the tumor. The authors designed engineered LPS-targeting fusion protein, the coding sequence of which was loaded into a lipid-protamine-DNA (LPD) system. When combined with an anti-PD-L1 antibody, the nanosystem not only prevented liver metastasis of CT26-FL3 cells but also remarkably inhibited CT26-FL3 tumor cell growth in the liver[289].
In addition to its contribution to the development of immunotherapeutic agents mentioned earlier, nanotechnology serves as a promising tool to enhance the efficacy of targeted therapy. For instance, erlotinib, an orally active inhibitor of EGFR, renders resistance during the treatment of CRC, leading to treatment failure. In addition, Javadi et al[290] developed a methoxy polyethylene glycol poly caprolactone loading curcumin and erlotinib. The nanomicelles were administrated in combination and they decreased the expression of αvβ3 integrin while increasing the expression of PDK4 in resistant SW480 cells. Resistance to cetuximab, which is another commonly used anti-EGFR antibody, was found to be reversed with the use of AuNPs (designed as cetuximab-conjugated AuNPs by Hallal et al[291]) The application of AuNPs loaded with cetuximab demonstrated an improved endocytosis effect and an enhanced inhibition of the downstream signaling pathway of HT29 cells relative to those with the application of cetuximab alone, suggesting their potential in reversing resistance to EGFR treatment in CRC[291].
Naseri et al[292] demonstrated that exosomes derived from CSC-enriched colonospheres (CSCenr-EXOs) significantly increased the IL-12/IL-10 ratio in supernatants of mature DCs. Furthermore, CSCenr-EXOs-loaded DCs effectively promoted T-cell proliferation. Hosseini et al[293] loaded exosomes isolated from CT-26 cell line with miR-34a mimic and found that these exosomes could diminish gene expressions related to invasion, angiogenesis and immune evasion and induce cytotoxic T cells in vivo.
Nanotechnology-targeted TME
In addition to targeting the immune environment in TME, CAFs should be considered a critical therapeutic target for overcoming the resistance of CRCs. Recently, the functions and regulatory mechanisms of CAFs were reviewed by Kobayashi, who illustrated in detail the clinical implications of using CAFs as biomarkers and targets for controlling gastrointestinal cancer[294]. The authors further recommended 6 strategies to target CAFs: Elimination of CAFs by using CAR T cells or vaccination; reprogramming of cancer-promoting CAFs (pCAFs) into cancer-retarding CAFs (rCAFs); conjugation of antibodies with cytokines; the administration of modified MSCs; blockade of biochemical signaling; ECM-targeting therapy by depleting hyaluronic acid (HA) or the inhibition of lysyl oxidase. As far as CRC is concerned, there has been an increasing interest along with considerable achievement in the application of fibroblast activation protein-targeted vaccination that increases chemotherapy sensitivity, as well as reprograming of CAFs with vitamin D[295,296]. Recently, Ji et al[297] designed a novel cleavable amphiphilic peptide (CAP) targeting FAP-α that can load hydrophobic drugs and release the cargo in the presence of FAP-α. The designed drug-delivery strategy, called the CAP-NPs, demonstrated promising specific targeting effects of tumors and achieved therapeutic efficacy for several tumor models[297]. Collectively, much remains to be done to optimally utilize nanotechnology targeting CAFs toward reversing CRC therapeutic resistance and controlling distant metastasis.
CONCLUSION
Resistance to therapies and metastasis are the most critical reasons that ultimately result in treatment failure for CRC patients. The application of nanotechnology in the fields of surgery, chemotherapy, radiotherapy, targeted therapy, and immunotherapy opens up broad prospects for overcoming drug resistance and controlling CRC metastasis owing to its unique targeted delivery, integration of multiple therapies, and synergistic therapeutic effects. For early-stage CRC, the modulation of the post-surgery microenvironment is worthy of investigation through the simultaneous implantation or spraying with nanomaterials-derived agents so as to improve the overall survival rate of the patients. As resistance to cytotoxic drugs is commonly encountered in a clinical setting, the codelivery of functionalized nanomaterials and natural phytochemicals is a novel alternative to restore the sensitivity of CRC cells. In radiotherapy, it is extremely promising to utilize the instinctive radiosensitivity of nanomaterials to reverse the hypoxic environment and combine it with immunotherapy to improve the overall efficacy of radiotherapy. In delivering gene therapy, nanotechnology offers distinct advantages that may be further studied to realize the specific antitumor effects. With the ongoing research on the immunotherapy mechanism behind CRC, it is necessary to work toward improving the immunomodulation effect of nanotechnology so as to convert non-reactive tumors into reactive tumors. In addition to targeting the immune cells in TME, CAFs can also be considered a promising target by nanotechnology to treat therapeutic resistance. Generally, treatment should not only target tumoral factors including genetic alterations, signal activation, or drug metabolites that lead to drug resistance but also pay attention to modulating the TME so as to eliminate the inhibitory factors and enhance powerful anticancer effects. Meanwhile, it is imperative to maintain a balance between the complexity of composition and structure and in the preparation and fabrication of nanoplatforms. Furthermore, better preclinical models are warranted to reflect the pathogenesis of CRC in order to achieve successful clinical translations of the emerging and established advancements in nanotechnology.
Footnotes
Conflict-of-interest statement: Authors declare no conflict of interests for this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: September 25, 2022
First decision: January 3, 2023
Article in press: March 21, 2023
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Belder N, Turkey; Portillo R, Czech Republic S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
Contributor Information
Sheng-Nan Ren, Nanomedicine and Translational Research Center, China-Japan Union Hospital of Jilin University, Changchun 130033, Jilin Province, China.
Zhan-Yi Zhang, Bethune Third Clinical Medical College, Jilin University, Changchun 130021, Jilin Province, China.
Rui-Jie Guo, Bethune Third Clinical Medical College, Jilin University, Changchun 130021, Jilin Province, China.
Da-Ren Wang, Bethune Third Clinical Medical College, Jilin University, Changchun 130021, Jilin Province, China.
Fang-Fang Chen, Nanomedicine and Translational Research Center, China-Japan Union Hospital of Jilin University, Changchun 130033, Jilin Province, China.
Xue-Bo Chen, Department of Gastrointestinal, Colorectal and Anal Surgery, China-Japan Union Hospital of Jilin University, Changchun 130033, Jilin Province, China. chenxb@jlu.edu.cn.
Xue-Dong Fang, Department of Gastrointestinal, Colorectal and Anal Surgery, China-Japan Union Hospital of Jilin University, Changchun 130033, Jilin Province, China.
References
- 1.Xi Y, Xu P. Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol. 2021;14:101174. doi: 10.1016/j.tranon.2021.101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merchant J, McArthur D, Ferguson H, Ramcharan S. Concepts and prospects of minimally invasive colorectal cancer surgery. Clin Radiol. 2021;76:889–895. doi: 10.1016/j.crad.2021.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Bregni G, Akin Telli T, Camera S, Deleporte A, Moretti L, Bali AM, Liberale G, Holbrechts S, Hendlisz A, Sclafani F. Adjuvant chemotherapy for rectal cancer: Current evidence and recommendations for clinical practice. Cancer Treat Rev. 2020;83:101948. doi: 10.1016/j.ctrv.2019.101948. [DOI] [PubMed] [Google Scholar]
- 4.Kuipers EJ, Grady WM, Lieberman D, Seufferlein T, Sung JJ, Boelens PG, van de Velde CJ, Watanabe T. Colorectal cancer. Nat Rev Dis Primers. 2015;1:15065. doi: 10.1038/nrdp.2015.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johdi NA, Sukor NF. Colorectal Cancer Immunotherapy: Options and Strategies. Front Immunol. 2020;11:1624. doi: 10.3389/fimmu.2020.01624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piawah S, Venook AP. Targeted therapy for colorectal cancer metastases: A review of current methods of molecularly targeted therapy and the use of tumor biomarkers in the treatment of metastatic colorectal cancer. Cancer. 2019;125:4139–4147. doi: 10.1002/cncr.32163. [DOI] [PubMed] [Google Scholar]
- 7.Guo Y, Wang M, Zou Y, Jin L, Zhao Z, Liu Q, Wang S, Li J. Mechanisms of chemotherapeutic resistance and the application of targeted nanoparticles for enhanced chemotherapy in colorectal cancer. J Nanobiotechnology. 2022;20:371. doi: 10.1186/s12951-022-01586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blondy S, David V, Verdier M, Mathonnet M, Perraud A, Christou N. 5-Fluorouracil resistance mechanisms in colorectal cancer: From classical pathways to promising processes. Cancer Sci. 2020;111:3142–3154. doi: 10.1111/cas.14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffith M, Mwenifumbo JC, Cheung PY, Paul JE, Pugh TJ, Tang MJ, Chittaranjan S, Morin RD, Asano JK, Ally AA, Miao L, Lee A, Chan SY, Taylor G, Severson T, Hou YC, Griffith OL, Cheng GS, Novik K, Moore R, Luk M, Owen D, Brown CJ, Morin GB, Gill S, Tai IT, Marra MA. Novel mRNA isoforms and mutations of uridine monophosphate synthetase and 5-fluorouracil resistance in colorectal cancer. Pharmacogenomics J. 2013;13:148–158. doi: 10.1038/tpj.2011.65. [DOI] [PubMed] [Google Scholar]
- 10.Chevalier F. Counteracting Radio-Resistance Using the Optimization of Radiotherapy. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21051767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Picard E, Verschoor CP, Ma GW, Pawelec G. Relationships Between Immune Landscapes, Genetic Subtypes and Responses to Immunotherapy in Colorectal Cancer. Front Immunol. 2020;11:369. doi: 10.3389/fimmu.2020.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fatfat Z, Fatfat M, Gali-Muhtasib H. Micelles as potential drug delivery systems for colorectal cancer treatment. World J Gastroenterol. 2022;28:2867–2880. doi: 10.3748/wjg.v28.i25.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu G, Shi C, Guo D, Wang L, Ling Y, Han X, Luo J. Functional-segregated coumarin-containing telodendrimer nanocarriers for efficient delivery of SN-38 for colon cancer treatment. Acta Biomater. 2015;21:85–98. doi: 10.1016/j.actbio.2015.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brunato S, Mastrotto F, Bellato F, Bastiancich C, Travanut A, Garofalo M, Mantovani G, Alexander C, Preat V, Salmaso S, Caliceti P. PEG-polyaminoacid based micelles for controlled release of doxorubicin: Rational design, safety and efficacy study. J Control Release. 2021;335:21–37. doi: 10.1016/j.jconrel.2021.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Wang K, Shen R, Meng T, Hu F, Yuan H. Nano-Drug Delivery Systems Based on Different Targeting Mechanisms in the Targeted Therapy of Colorectal Cancer. Molecules. 2022;27 doi: 10.3390/molecules27092981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guan X, Sun L, Shen Y, Jin F, Bo X, Zhu C, Han X, Li X, Chen Y, Xu H, Yue W. Nanoparticle-enhanced radiotherapy synergizes with PD-L1 blockade to limit post-surgical cancer recurrence and metastasis. Nat Commun. 2022;13:2834. doi: 10.1038/s41467-022-30543-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanati S, Taghavi S, Abnous K, Taghdisi SM, Babaei M, Ramezani M, Alibolandi M. Fabrication of anionic dextran-coated micelles for aptamer targeted delivery of camptothecin and survivin-shRNA to colon adenocarcinoma. Gene Ther. 2022;29:55–68. doi: 10.1038/s41434-021-00234-0. [DOI] [PubMed] [Google Scholar]
- 18.Guerra DB, Oliveira EMN, Sonntag AR, Sbaraine P, Fay AP, Morrone FB, Papaléo RM. Intercomparison of radiosensitization induced by gold and iron oxide nanoparticles in human glioblastoma cells irradiated by 6 MV photons. Sci Rep. 2022;12:9602. doi: 10.1038/s41598-022-13368-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H, Wang M, Huang B, Zhu SW, Zhou JJ, Chen DR, Cui R, Zhang M, Sun ZJ. Theranostic near-infrared-IIb emitting nanoprobes for promoting immunogenic radiotherapy and abscopal effects against cancer metastasis. Nat Commun. 2021;12:7149. doi: 10.1038/s41467-021-27485-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji G, Zhang Y, Si X, Yao H, Ma S, Xu Y, Zhao J, Ma C, He C, Tang Z, Fang X, Song W, Chen X. Biopolymer Immune Implants' Sequential Activation of Innate and Adaptive Immunity for Colorectal Cancer Postoperative Immunotherapy. Adv Mater. 2021;33:e2004559. doi: 10.1002/adma.202004559. [DOI] [PubMed] [Google Scholar]
- 21.Si X, Ji G, Ma S, Xu Y, Zhao J, Zhang Y, Huang Z, Tang Z, Song W, Chen X. In-Situ-Sprayed Dual-Functional Immunotherapeutic Gel for Colorectal Cancer Postsurgical Treatment. Adv Healthc Mater. 2021;10:e2100862. doi: 10.1002/adhm.202100862. [DOI] [PubMed] [Google Scholar]
- 22.Liu J, Li L, Zhang B, Xu ZP. MnO(2)-shelled Doxorubicin/Curcumin nanoformulation for enhanced colorectal cancer chemo-immunotherapy. J Colloid Interface Sci. 2022;617:315–325. doi: 10.1016/j.jcis.2022.02.132. [DOI] [PubMed] [Google Scholar]
- 23.De Mattia E, Cecchin E, Toffoli G. Pharmacogenomics of intrinsic and acquired pharmacoresistance in colorectal cancer: Toward targeted personalized therapy. Drug Resist Updat. 2015;20:39–70. doi: 10.1016/j.drup.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Malet-Martino M, Martino R. Clinical studies of three oral prodrugs of 5-fluorouracil (capecitabine, UFT, S-1): a review. Oncologist. 2002;7:288–323. doi: 10.1634/theoncologist.7-4-288. [DOI] [PubMed] [Google Scholar]
- 25.Kosuri KV, Wu X, Wang L, Villalona-Calero MA, Otterson GA. An epigenetic mechanism for capecitabine resistance in mesothelioma. Biochem Biophys Res Commun. 2010;391:1465–1470. doi: 10.1016/j.bbrc.2009.12.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kciuk M, Marciniak B, Kontek R. Irinotecan-Still an Important Player in Cancer Chemotherapy: A Comprehensive Overview. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21144919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaojun C, Li H, Haixin H, Guisheng L. Expression of Topoisomerase 1 and carboxylesterase 2 correlates with irinotecan treatment response in metastatic colorectal cancer. Cancer Biol Ther. 2018;19:153–159. doi: 10.1080/15384047.2017.1414754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soong R, Shah N, Salto-Tellez M, Tai BC, Soo RA, Han HC, Ng SS, Tan WL, Zeps N, Joseph D, Diasio RB, Iacopetta B. Prognostic significance of thymidylate synthase, dihydropyrimidine dehydrogenase and thymidine phosphorylase protein expression in colorectal cancer patients treated with or without 5-fluorouracil-based chemotherapy. Ann Oncol. 2008;19:915–919. doi: 10.1093/annonc/mdm599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Man FM, Goey AKL, van Schaik RHN, Mathijssen RHJ, Bins S. Individualization of Irinotecan Treatment: A Review of Pharmacokinetics, Pharmacodynamics, and Pharmacogenetics. Clin Pharmacokinet. 2018;57:1229–1254. doi: 10.1007/s40262-018-0644-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kong R, Liu T, Zhu X, Ahmad S, Williams AL, Phan AT, Zhao H, Scott JE, Yeh LA, Wong ST. Old drug new use--amoxapine and its metabolites as potent bacterial β-glucuronidase inhibitors for alleviating cancer drug toxicity. Clin Cancer Res. 2014;20:3521–3530. doi: 10.1158/1078-0432.CCR-14-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.International Transporter Consortium. Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM, Hoffmaster KA, Ishikawa T, Keppler D, Kim RB, Lee CA, Niemi M, Polli JW, Sugiyama Y, Swaan PW, Ware JA, Wright SH, Yee SW, Zamek-Gliszczynski MJ, Zhang L. Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9:215–236. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Du J, He Y, Li P, Wu W, Chen Y, Ruan H. IL-8 regulates the doxorubicin resistance of colorectal cancer cells via modulation of multidrug resistance 1 (MDR1) Cancer Chemother Pharmacol. 2018;81:1111–1119. doi: 10.1007/s00280-018-3584-x. [DOI] [PubMed] [Google Scholar]
- 33.Gao R, Fang C, Xu J, Tan H, Li P, Ma L. LncRNA CACS15 contributes to oxaliplatin resistance in colorectal cancer by positively regulating ABCC1 through sponging miR-145. Arch Biochem Biophys. 2019;663:183–191. doi: 10.1016/j.abb.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Wang B, Lu FY, Shi RH, Feng YD, Zhao XD, Lu ZP, Xiao L, Zhou GQ, Qiu JM, Cheng CE. MiR-26b regulates 5-FU-resistance in human colorectal cancer via down-regulation of Pgp. Am J Cancer Res. 2018;8:2518–2527. [PMC free article] [PubMed] [Google Scholar]
- 35.Mosca L, Pagano M, Borzacchiello L, Mele L, Russo A, Russo G, Cacciapuoti G, Porcelli M. S-Adenosylmethionine Increases the Sensitivity of Human Colorectal Cancer Cells to 5-Fluorouracil by Inhibiting P-Glycoprotein Expression and NF-κB Activation. Int J Mol Sci. 2021;22 doi: 10.3390/ijms22179286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dang Y, Liu F, Zhao Y. P-Gp and TOPO II Expression and Their Clinical Significance in Colon Cancer. Ann Clin Lab Sci. 2020;50:584–590. [PubMed] [Google Scholar]
- 37.Wang Q, Shen X, Chen G, Du J. Drug Resistance in Colorectal Cancer: From Mechanism to Clinic. Cancers (Basel) 2022;14 doi: 10.3390/cancers14122928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grogan L, Sotos GA, Allegra CJ. Leucovorin modulation of fluorouracil. Oncology (Williston Park) 1993;7:63–72; discussion 75. [PubMed] [Google Scholar]
- 39.Xu Y, Villalona-Calero MA. Irinotecan: mechanisms of tumor resistance and novel strategies for modulating its activity. Ann Oncol. 2002;13:1841–1851. doi: 10.1093/annonc/mdf337. [DOI] [PubMed] [Google Scholar]
- 40.Wu Y, Wang KY, Li Z, Liu YP, Izumi H, Uramoto H, Nakayama Y, Ito K, Kohno K. Y-box binding protein 1 enhances DNA topoisomerase 1 activity and sensitivity to camptothecin via direct interaction. J Exp Clin Cancer Res. 2014;33:112. doi: 10.1186/s13046-014-0112-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bugter JM, Fenderico N, Maurice MM. Mutations and mechanisms of WNT pathway tumour suppressors in cancer. Nat Rev Cancer. 2021;21:5–21. doi: 10.1038/s41568-020-00307-z. [DOI] [PubMed] [Google Scholar]
- 42.Li Q, Yang T, Li D, Ding F, Bai G, Wang W, Sun H. Knockdown of aquaporin-5 sensitizes colorectal cancer cells to 5-fluorouracil via inhibition of the Wnt-β-catenin signaling pathway. Biochem Cell Biol. 2018;96:572–579. doi: 10.1139/bcb-2017-0162. [DOI] [PubMed] [Google Scholar]
- 43.Spitzner M, Roesler B, Bielfeld C, Emons G, Gaedcke J, Wolff HA, Rave-Fränk M, Kramer F, Beissbarth T, Kitz J, Wienands J, Ghadimi BM, Ebner R, Ried T, Grade M. STAT3 inhibition sensitizes colorectal cancer to chemoradiotherapy in vitro and in vivo. Int J Cancer. 2014;134:997–1007. doi: 10.1002/ijc.28429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burke JD, Young HA. IFN-γ: A cytokine at the right time, is in the right place. Semin Immunol. 2019;43:101280. doi: 10.1016/j.smim.2019.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shin DS, Zaretsky JM, Escuin-Ordinas H, Garcia-Diaz A, Hu-Lieskovan S, Kalbasi A, Grasso CS, Hugo W, Sandoval S, Torrejon DY, Palaskas N, Rodriguez GA, Parisi G, Azhdam A, Chmielowski B, Cherry G, Seja E, Berent-Maoz B, Shintaku IP, Le DT, Pardoll DM, Diaz LA Jr, Tumeh PC, Graeber TG, Lo RS, Comin-Anduix B, Ribas A. Primary Resistance to PD-1 Blockade Mediated by JAK1/2 Mutations. Cancer Discov. 2017;7:188–201. doi: 10.1158/2159-8290.CD-16-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brunen D, Willems SM, Kellner U, Midgley R, Simon I, Bernards R. TGF-β: an emerging player in drug resistance. Cell Cycle. 2013;12:2960–2968. doi: 10.4161/cc.26034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang S, Hölzel M, Knijnenburg T, Schlicker A, Roepman P, McDermott U, Garnett M, Grernrum W, Sun C, Prahallad A, Groenendijk FH, Mittempergher L, Nijkamp W, Neefjes J, Salazar R, Ten Dijke P, Uramoto H, Tanaka F, Beijersbergen RL, Wessels LF, Bernards R. MED12 controls the response to multiple cancer drugs through regulation of TGF-β receptor signaling. Cell. 2012;151:937–950. doi: 10.1016/j.cell.2012.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quan Q, Zhong F, Wang X, Chen K, Guo L. PAR2 Inhibition Enhanced the Sensitivity of Colorectal Cancer Cells to 5-FU and Reduced EMT Signaling. Oncol Res. 2019;27:779–788. doi: 10.3727/096504018X15442985680348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murugan AK. mTOR: Role in cancer, metastasis and drug resistance. Semin Cancer Biol. 2019;59:92–111. doi: 10.1016/j.semcancer.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 50.Liu B, Liu Y, Zhao L, Pan Y, Shan Y, Li Y, Jia L. Upregulation of microRNA-135b and microRNA-182 promotes chemoresistance of colorectal cancer by targeting ST6GALNAC2 via PI3K/AKT pathway. Mol Carcinog. 2017;56:2669–2680. doi: 10.1002/mc.22710. [DOI] [PubMed] [Google Scholar]
- 51.Sun LT, Zhang LY, Shan FY, Shen MH, Ruan SM. Jiedu Sangen decoction inhibits chemoresistance to 5-fluorouracil of colorectal cancer cells by suppressing glycolysis via PI3K/AKT/HIF-1α signaling pathway. Chin J Nat Med. 2021;19:143–152. doi: 10.1016/S1875-5364(21)60015-8. [DOI] [PubMed] [Google Scholar]
- 52.Lu LL, Chen XH, Zhang G, Liu ZC, Wu N, Wang H, Qi YF, Wang HS, Cai SH, Du J. CCL21 Facilitates Chemoresistance and Cancer Stem Cell-Like Properties of Colorectal Cancer Cells through AKT/GSK-3β/Snail Signals. Oxid Med Cell Longev. 2016;2016:5874127. doi: 10.1155/2016/5874127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang H, Tian T, Zhang J. Tumor-Associated Macrophages (TAMs) in Colorectal Cancer (CRC): From Mechanism to Therapy and Prognosis. Int J Mol Sci. 2021;22 doi: 10.3390/ijms22168470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liao W, Shi M, Zeng D, Huang N, Wang Y, Li J, Wang C, Rong X, Fang Y, Zheng S, Xue Y, Wang J, Guo J. Tumor and microenvironment evolution during chemotherapy combine with bevacizumab in colorectal cancer liver metastasis. J Clin Oncol. 2019;37:3568. [Google Scholar]
- 55.Gupta M, Smith T, Bshara W, Terhune J, Attwood K, Mukherjee S, Iyer RV, Boland PM. The impact of neoadjuvant chemotherapy (NACT) on the tumor immune microenvironment (TME) in patients with colorectal cancer with liver metastases (CRCLM) J Clin Oncol. 2021;39:124. [Google Scholar]
- 56.Chen H, Yao J, Bao R, Dong Y, Zhang T, Du Y, Wang G, Ni D, Xun Z, Niu X, Ye Y, Li HB. Cross-talk of four types of RNA modification writers defines tumor microenvironment and pharmacogenomic landscape in colorectal cancer. Mol Cancer. 2021;20:29. doi: 10.1186/s12943-021-01322-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu X, Tian X, Ji L, Zhang X, Cao Y, Shen C, Hu Y, Wong JWH, Fang JY, Hong J, Chen H. A tumor microenvironment-specific gene expression signature predicts chemotherapy resistance in colorectal cancer patients. NPJ Precis Oncol. 2021;5:7. doi: 10.1038/s41698-021-00142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Escalante PI, Quiñones LA, Contreras HR. Epithelial-Mesenchymal Transition and MicroRNAs in Colorectal Cancer Chemoresistance to FOLFOX. Pharmaceutics. 2021;13 doi: 10.3390/pharmaceutics13010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luo JW, Wang CM, Su JW, Yi TZ, Tang SH. CUL4B increases platinum-based drug resistance in colorectal cancer through EMT: A study in its mechanism. J Cell Mol Med. 2022;26:5767–5778. doi: 10.1111/jcmm.17585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang J, Miller Z, Musich PR, Thomas AE, Yao ZQ, Xie Q, Howe PH, Jiang Y. DSTYK Promotes Metastasis and Chemoresistance via EMT in Colorectal Cancer. Front Pharmacol. 2020;11:1250. doi: 10.3389/fphar.2020.01250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu Y, Huang S, Chen S, Chen J, Wang Z, Wang Y, Zheng H. SOX2 promotes chemoresistance, cancer stem cells properties, and epithelial-mesenchymal transition by β-catenin and Beclin1/autophagy signaling in colorectal cancer. Cell Death Dis. 2021;12:449. doi: 10.1038/s41419-021-03733-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Siveen KS, Raza A, Ahmed EI, Khan AQ, Prabhu KS, Kuttikrishnan S, Mateo JM, Zayed H, Rasul K, Azizi F, Dermime S, Steinhoff M, Uddin S. The Role of Extracellular Vesicles as Modulators of the Tumor Microenvironment, Metastasis and Drug Resistance in Colorectal Cancer. Cancers (Basel) 2019;11 doi: 10.3390/cancers11060746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Campos A, Sharma S, Obermair A, Salomon C. Extracellular Vesicle-Associated miRNAs and Chemoresistance: A Systematic Review. Cancers (Basel) 2021;13 doi: 10.3390/cancers13184608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hu YB, Yan C, Mu L, Mi YL, Zhao H, Hu H, Li XL, Tao DD, Wu YQ, Gong JP, Qin JC. Exosomal Wnt-induced dedifferentiation of colorectal cancer cells contributes to chemotherapy resistance. Oncogene. 2019;38:1951–1965. doi: 10.1038/s41388-018-0557-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang X, Zhang H, Yang H, Bai M, Ning T, Deng T, Liu R, Fan Q, Zhu K, Li J, Zhan Y, Ying G, Ba Y. Exosome-delivered circRNA promotes glycolysis to induce chemoresistance through the miR-122-PKM2 axis in colorectal cancer. Mol Oncol. 2020;14:539–555. doi: 10.1002/1878-0261.12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shedden K, Xie XT, Chandaroy P, Chang YT, Rosania GR. Expulsion of small molecules in vesicles shed by cancer cells: association with gene expression and chemosensitivity profiles. Cancer Res. 2003;63:4331–4337. [PubMed] [Google Scholar]
- 67.Robey RW, Pluchino KM, Hall MD, Fojo AT, Bates SE, Gottesman MM. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat Rev Cancer. 2018;18:452–464. doi: 10.1038/s41568-018-0005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mashouri L, Yousefi H, Aref AR, Ahadi AM, Molaei F, Alahari SK. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol Cancer. 2019;18:75. doi: 10.1186/s12943-019-0991-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maacha S, Bhat AA, Jimenez L, Raza A, Haris M, Uddin S, Grivel JC. Extracellular vesicles-mediated intercellular communication: roles in the tumor microenvironment and anti-cancer drug resistance. Mol Cancer. 2019;18:55. doi: 10.1186/s12943-019-0965-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Namee NM, O'Driscoll L. Extracellular vesicles and anti-cancer drug resistance. Biochim Biophys Acta Rev Cancer. 2018;1870:123–136. doi: 10.1016/j.bbcan.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 71.Woodward WA, Chen MS, Behbod F, Alfaro MP, Buchholz TA, Rosen JM. WNT/beta-catenin mediates radiation resistance of mouse mammary progenitor cells. Proc Natl Acad Sci U S A. 2007;104:618–623. doi: 10.1073/pnas.0606599104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 73.Lomonaco SL, Finniss S, Xiang C, Decarvalho A, Umansky F, Kalkanis SN, Mikkelsen T, Brodie C. The induction of autophagy by gamma-radiation contributes to the radioresistance of glioma stem cells. Int J Cancer. 2009;125:717–722. doi: 10.1002/ijc.24402. [DOI] [PubMed] [Google Scholar]
- 74.Olivares-Urbano MA, Griñán-Lisón C, Marchal JA, Núñez MI. CSC Radioresistance: A Therapeutic Challenge to Improve Radiotherapy Effectiveness in Cancer. Cells. 2020;9 doi: 10.3390/cells9071651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Batlle E, Clevers H. Cancer stem cells revisited. Nat Med. 2017;23:1124–1134. doi: 10.1038/nm.4409. [DOI] [PubMed] [Google Scholar]
- 76.Yang L, Shi P, Zhao G, Xu J, Peng W, Zhang J, Zhang G, Wang X, Dong Z, Chen F, Cui H. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct Target Ther. 2020;5:8. doi: 10.1038/s41392-020-0110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao Y, Tao L, Yi J, Song H, Chen L. The Role of Canonical Wnt Signaling in Regulating Radioresistance. Cell Physiol Biochem. 2018;48:419–432. doi: 10.1159/000491774. [DOI] [PubMed] [Google Scholar]
- 78.Schulz A, Meyer F, Dubrovska A, Borgmann K. Cancer Stem Cells and Radioresistance: DNA Repair and Beyond. Cancers (Basel) 2019;11 doi: 10.3390/cancers11060862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee SY, Jeong EK, Ju MK, Jeon HM, Kim MY, Kim CH, Park HG, Han SI, Kang HS. Induction of metastasis, cancer stem cell phenotype, and oncogenic metabolism in cancer cells by ionizing radiation. Mol Cancer. 2017;16:10. doi: 10.1186/s12943-016-0577-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chao HX, Poovey CE, Privette AA, Grant GD, Chao HY, Cook JG, Purvis JE. Orchestration of DNA Damage Checkpoint Dynamics across the Human Cell Cycle. Cell Syst. 2017;5:445–459.e5. doi: 10.1016/j.cels.2017.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang H, Wang Z, Li Y, Lu T, Hu G. Silencing Snail Reverses Epithelial-Mesenchymal Transition and Increases Radiosensitivity in Hypopharyngeal Carcinoma. Onco Targets Ther. 2020;13:497–511. doi: 10.2147/OTT.S237410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Krause M, Dubrovska A, Linge A, Baumann M. Cancer stem cells: Radioresistance, prediction of radiotherapy outcome and specific targets for combined treatments. Adv Drug Deliv Rev. 2017;109:63–73. doi: 10.1016/j.addr.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 83.Najafi M, Mortezaee K, Majidpoor J. Cancer stem cell (CSC) resistance drivers. Life Sci. 2019;234:116781. doi: 10.1016/j.lfs.2019.116781. [DOI] [PubMed] [Google Scholar]
- 84.Barker HE, Paget JT, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer. 2015;15:409–425. doi: 10.1038/nrc3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Barcellos-Hoff MH, Ravani SA. Irradiated mammary gland stroma promotes the expression of tumorigenic potential by unirradiated epithelial cells. Cancer Res. 2000;60:1254–1260. [PubMed] [Google Scholar]
- 86.Heckmann M, Douwes K, Peter R, Degitz K. Vascular activation of adhesion molecule mRNA and cell surface expression by ionizing radiation. Exp Cell Res. 1998;238:148–154. doi: 10.1006/excr.1997.3826. [DOI] [PubMed] [Google Scholar]
- 87.Langley RE, Bump EA, Quartuccio SG, Medeiros D, Braunhut SJ. Radiation-induced apoptosis in microvascular endothelial cells. Br J Cancer. 1997;75:666–672. doi: 10.1038/bjc.1997.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ritter V, Krautter F, Klein D, Jendrossek V, Rudner J. Bcl-2/Bcl-xL inhibitor ABT-263 overcomes hypoxia-driven radioresistence and improves radiotherapy. Cell Death Dis. 2021;12:694. doi: 10.1038/s41419-021-03971-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wouters BG, Koritzinsky M, Chiu RK, Theys J, Buijsen J, Lambin P. Modulation of cell death in the tumor microenvironment. Semin Radiat Oncol. 2003;13:31–41. doi: 10.1053/srao.2003.50004. [DOI] [PubMed] [Google Scholar]
- 90.Mantoni TS, Lunardi S, Al-Assar O, Masamune A, Brunner TB. Pancreatic stellate cells radioprotect pancreatic cancer cells through β1-integrin signaling. Cancer Res. 2011;71:3453–3458. doi: 10.1158/0008-5472.CAN-10-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Carracedo S, Lu N, Popova SN, Jonsson R, Eckes B, Gullberg D. The fibroblast integrin alpha11beta1 is induced in a mechanosensitive manner involving activin A and regulates myofibroblast differentiation. J Biol Chem. 2010;285:10434–10445. doi: 10.1074/jbc.M109.078766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Finkelstein SE, Iclozan C, Bui MM, Cotter MJ, Ramakrishnan R, Ahmed J, Noyes DR, Cheong D, Gonzalez RJ, Heysek RV, Berman C, Lenox BC, Janssen W, Zager JS, Sondak VK, Letson GD, Antonia SJ, Gabrilovich DI. Combination of external beam radiotherapy (EBRT) with intratumoral injection of dendritic cells as neo-adjuvant treatment of high-risk soft tissue sarcoma patients. Int J Radiat Oncol Biol Phys. 2012;82:924–932. doi: 10.1016/j.ijrobp.2010.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kachikwu EL, Iwamoto KS, Liao YP, DeMarco JJ, Agazaryan N, Economou JS, McBride WH, Schaue D. Radiation enhances regulatory T cell representation. Int J Radiat Oncol Biol Phys. 2011;81:1128–1135. doi: 10.1016/j.ijrobp.2010.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Qu Y, Jin S, Zhang A, Zhang B, Shi X, Wang J, Zhao Y. Gamma-ray resistance of regulatory CD4+CD25+Foxp3+ T cells in mice. Radiat Res. 2010;173:148–157. doi: 10.1667/RR0978.1. [DOI] [PubMed] [Google Scholar]
- 95.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu YX. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687–695. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Verbrugge I, Hagekyriakou J, Sharp LL, Galli M, West A, McLaughlin NM, Duret H, Yagita H, Johnstone RW, Smyth MJ, Haynes NM. Radiotherapy increases the permissiveness of established mammary tumors to rejection by immunomodulatory antibodies. Cancer Res. 2012;72:3163–3174. doi: 10.1158/0008-5472.CAN-12-0210. [DOI] [PubMed] [Google Scholar]
- 97.Marabelle A, Filatenkov A, Sagiv-Barfi I, Kohrt H. Radiotherapy and toll-like receptor agonists. Semin Radiat Oncol. 2015;25:34–39. doi: 10.1016/j.semradonc.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 98.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA Jr. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, Morse MA, Van Cutsem E, McDermott R, Hill A, Sawyer MB, Hendlisz A, Neyns B, Svrcek M, Moss RA, Ledeine JM, Cao ZA, Kamble S, Kopetz S, André T. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J Clin Oncol. 2018;36:773–779. doi: 10.1200/JCO.2017.76.9901. [DOI] [PubMed] [Google Scholar]
- 100.Xiao Q, Wu J, Wang WJ, Chen S, Zheng Y, Yu X, Meeth K, Sahraei M, Bothwell ALM, Chen L, Bosenberg M, Chen J, Sexl V, Sun L, Li L, Tang W, Wu D. DKK2 imparts tumor immunity evasion through β-catenin-independent suppression of cytotoxic immune-cell activation. Nat Med. 2018;24:262–270. doi: 10.1038/nm.4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Grasso CS, Giannakis M, Wells DK, Hamada T, Mu XJ, Quist M, Nowak JA, Nishihara R, Qian ZR, Inamura K, Morikawa T, Nosho K, Abril-Rodriguez G, Connolly C, Escuin-Ordinas H, Geybels MS, Grady WM, Hsu L, Hu-Lieskovan S, Huyghe JR, Kim YJ, Krystofinski P, Leiserson MDM, Montoya DJ, Nadel BB, Pellegrini M, Pritchard CC, Puig-Saus C, Quist EH, Raphael BJ, Salipante SJ, Shin DS, Shinbrot E, Shirts B, Shukla S, Stanford JL, Sun W, Tsoi J, Upfill-Brown A, Wheeler DA, Wu CJ, Yu M, Zaidi SH, Zaretsky JM, Gabriel SB, Lander ES, Garraway LA, Hudson TJ, Fuchs CS, Ribas A, Ogino S, Peters U. Genetic Mechanisms of Immune Evasion in Colorectal Cancer. Cancer Discov. 2018;8:730–749. doi: 10.1158/2159-8290.CD-17-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kloor M, Michel S, von Knebel Doeberitz M. Immune evasion of microsatellite unstable colorectal cancers. Int J Cancer. 2010;127:1001–1010. doi: 10.1002/ijc.25283. [DOI] [PubMed] [Google Scholar]
- 103.Kloor M, Becker C, Benner A, Woerner SM, Gebert J, Ferrone S, von Knebel Doeberitz M. Immunoselective pressure and human leukocyte antigen class I antigen machinery defects in microsatellite unstable colorectal cancers. Cancer Res. 2005;65:6418–6424. doi: 10.1158/0008-5472.CAN-05-0044. [DOI] [PubMed] [Google Scholar]
- 104.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, Wong F, Azad NS, Rucki AA, Laheru D, Donehower R, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Greten TF, Duffy AG, Ciombor KK, Eyring AD, Lam BH, Joe A, Kang SP, Holdhoff M, Danilova L, Cope L, Meyer C, Zhou S, Goldberg RM, Armstrong DK, Bever KM, Fader AN, Taube J, Housseau F, Spetzler D, Xiao N, Pardoll DM, Papadopoulos N, Kinzler KW, Eshleman JR, Vogelstein B, Anders RA, Diaz LA Jr. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Janikovits J, Müller M, Krzykalla J, Körner S, Echterdiek F, Lahrmann B, Grabe N, Schneider M, Benner A, Doeberitz MVK, Kloor M. High numbers of PDCD1 (PD-1)-positive T cells and B2M mutations in microsatellite-unstable colorectal cancer. Oncoimmunology. 2018;7:e1390640. doi: 10.1080/2162402X.2017.1390640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Giannakis M, Mu XJ, Shukla SA, Qian ZR, Cohen O, Nishihara R, Bahl S, Cao Y, Amin-Mansour A, Yamauchi M, Sukawa Y, Stewart C, Rosenberg M, Mima K, Inamura K, Nosho K, Nowak JA, Lawrence MS, Giovannucci EL, Chan AT, Ng K, Meyerhardt JA, Van Allen EM, Getz G, Gabriel SB, Lander ES, Wu CJ, Fuchs CS, Ogino S, Garraway LA. Genomic Correlates of Immune-Cell Infiltrates in Colorectal Carcinoma. Cell Rep. 2016;15:857–865. doi: 10.1016/j.celrep.2016.03.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ozcan M, Janikovits J, von Knebel Doeberitz M, Kloor M. Complex pattern of immune evasion in MSI colorectal cancer. Oncoimmunology. 2018;7:e1445453. doi: 10.1080/2162402X.2018.1445453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liao W, Overman MJ, Boutin AT, Shang X, Zhao D, Dey P, Li J, Wang G, Lan Z, Tang M, Jiang S, Ma X, Chen P, Katkhuda R, Korphaisarn K, Chakravarti D, Chang A, Spring DJ, Chang Q, Zhang J, Maru DM, Maeda DY, Zebala JA, Kopetz S, Wang YA, DePinho RA. KRAS-IRF2 Axis Drives Immune Suppression and Immune Therapy Resistance in Colorectal Cancer. Cancer Cell. 2019;35:559–572.e7. doi: 10.1016/j.ccell.2019.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 110.Galon J, Mlecnik B, Bindea G, Angell HK, Berger A, Lagorce C, Lugli A, Zlobec I, Hartmann A, Bifulco C, Nagtegaal ID, Palmqvist R, Masucci GV, Botti G, Tatangelo F, Delrio P, Maio M, Laghi L, Grizzi F, Asslaber M, D'Arrigo C, Vidal-Vanaclocha F, Zavadova E, Chouchane L, Ohashi PS, Hafezi-Bakhtiari S, Wouters BG, Roehrl M, Nguyen L, Kawakami Y, Hazama S, Okuno K, Ogino S, Gibbs P, Waring P, Sato N, Torigoe T, Itoh K, Patel PS, Shukla SN, Wang Y, Kopetz S, Sinicrope FA, Scripcariu V, Ascierto PA, Marincola FM, Fox BA, Pagès F. Towards the introduction of the 'Immunoscore' in the classification of malignant tumours. J Pathol. 2014;232:199–209. doi: 10.1002/path.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mlecnik B, Tosolini M, Kirilovsky A, Berger A, Bindea G, Meatchi T, Bruneval P, Trajanoski Z, Fridman WH, Pagès F, Galon J. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol. 2011;29:610–618. doi: 10.1200/JCO.2010.30.5425. [DOI] [PubMed] [Google Scholar]
- 112.Yomoda T, Sudo T, Kawahara A, Shigaki T, Shimomura S, Tajiri K, Nagasu S, Fujita F, Kinugasa T, Akagi Y. The Immunoscore is a Superior Prognostic Tool in Stages II and III Colorectal Cancer and is Significantly Correlated with Programmed Death-Ligand 1 (PD-L1) Expression on Tumor-Infiltrating Mononuclear Cells. Ann Surg Oncol. 2019;26:415–424. doi: 10.1245/s10434-018-07110-z. [DOI] [PubMed] [Google Scholar]
- 113.Ishii H, Azuma K, Kawahara A, Matsuo N, Tokito T, Kinoshita T, Yamada K, Sasada T, Akiba J, Hoshino T. Programmed cell death-ligand 1 expression and immunoscore in stage II and III non-small cell lung cancer patients receiving adjuvant chemotherapy. Oncotarget. 2017;8:61618–61625. doi: 10.18632/oncotarget.18651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Guinney J, Dienstmann R, Wang X, de Reyniès A, Schlicker A, Soneson C, Marisa L, Roepman P, Nyamundanda G, Angelino P, Bot BM, Morris JS, Simon IM, Gerster S, Fessler E, De Sousa E Melo F, Missiaglia E, Ramay H, Barras D, Homicsko K, Maru D, Manyam GC, Broom B, Boige V, Perez-Villamil B, Laderas T, Salazar R, Gray JW, Hanahan D, Tabernero J, Bernards R, Friend SH, Laurent-Puig P, Medema JP, Sadanandam A, Wessels L, Delorenzi M, Kopetz S, Vermeulen L, Tejpar S. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21:1350–1356. doi: 10.1038/nm.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, Schrock A, Campbell B, Shlien A, Chmielecki J, Huang F, He Y, Sun J, Tabori U, Kennedy M, Lieber DS, Roels S, White J, Otto GA, Ross JS, Garraway L, Miller VA, Stephens PJ, Frampton GM. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9:34. doi: 10.1186/s13073-017-0424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen YP, Zhang Y, Lv JW, Li YQ, Wang YQ, He QM, Yang XJ, Sun Y, Mao YP, Yun JP, Liu N, Ma J. Genomic Analysis of Tumor Microenvironment Immune Types across 14 Solid Cancer Types: Immunotherapeutic Implications. Theranostics. 2017;7:3585–3594. doi: 10.7150/thno.21471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 118.Becht E, de Reyniès A, Giraldo NA, Pilati C, Buttard B, Lacroix L, Selves J, Sautès-Fridman C, Laurent-Puig P, Fridman WH. Immune and Stromal Classification of Colorectal Cancer Is Associated with Molecular Subtypes and Relevant for Precision Immunotherapy. Clin Cancer Res. 2016;22:4057–4066. doi: 10.1158/1078-0432.CCR-15-2879. [DOI] [PubMed] [Google Scholar]
- 119.Lu W, Yu W, He J, Liu W, Yang J, Lin X, Zhang Y, Wang X, Jiang W, Luo J, Zhang Q, Yang H, Peng S, Yi Z, Ren S, Chen J, Siwko S, Nussinov R, Cheng F, Zhang H, Liu M. Reprogramming immunosuppressive myeloid cells facilitates immunotherapy for colorectal cancer. EMBO Mol Med. 2021;13:e12798. doi: 10.15252/emmm.202012798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Van der Jeught K, Xu HC, Li YJ, Lu XB, Ji G. Drug resistance and new therapies in colorectal cancer. World J Gastroenterol. 2018;24:3834–3848. doi: 10.3748/wjg.v24.i34.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Goc J, Lv M, Bessman NJ, Flamar AL, Sahota S, Suzuki H, Teng F, Putzel GG JRI Live Cell Bank, Eberl G, Withers DR, Arthur JC, Shah MA, Sonnenberg GF. Dysregulation of ILC3s unleashes progression and immunotherapy resistance in colon cancer. Cell. 2021;184:5015–5030.e16. doi: 10.1016/j.cell.2021.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen L, Gibbons DL, Goswami S, Cortez MA, Ahn YH, Byers LA, Zhang X, Yi X, Dwyer D, Lin W, Diao L, Wang J, Roybal J, Patel M, Ungewiss C, Peng D, Antonia S, Mediavilla-Varela M, Robertson G, Suraokar M, Welsh JW, Erez B, Wistuba II, Chen L, Wang S, Ullrich SE, Heymach JV, Kurie JM, Qin FX. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat Commun. 2014;5:5241. doi: 10.1038/ncomms6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kudo-Saito C, Shirako H, Takeuchi T, Kawakami Y. Cancer metastasis is accelerated through immunosuppression during Snail-induced EMT of cancer cells. Cancer Cell. 2009;15:195–206. doi: 10.1016/j.ccr.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 124.Horn LA, Fousek K, Palena C. Tumor Plasticity and Resistance to Immunotherapy. Trends Cancer. 2020;6:432–441. doi: 10.1016/j.trecan.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Frank MH, Wilson BJ, Gold JS, Frank NY. Clinical Implications of Colorectal Cancer Stem Cells in the Age of Single-Cell Omics and Targeted Therapies. Gastroenterology. 2021;160:1947–1960. doi: 10.1053/j.gastro.2020.12.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Khambata-Ford S, Garrett CR, Meropol NJ, Basik M, Harbison CT, Wu S, Wong TW, Huang X, Takimoto CH, Godwin AK, Tan BR, Krishnamurthi SS, Burris HA 3rd, Poplin EA, Hidalgo M, Baselga J, Clark EA, Mauro DJ. Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J Clin Oncol. 2007;25:3230–3237. doi: 10.1200/JCO.2006.10.5437. [DOI] [PubMed] [Google Scholar]
- 127.De Roock W, Piessevaux H, De Schutter J, Janssens M, De Hertogh G, Personeni N, Biesmans B, Van Laethem JL, Peeters M, Humblet Y, Van Cutsem E, Tejpar S. KRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectal cancer treated with cetuximab. Ann Oncol. 2008;19:508–515. doi: 10.1093/annonc/mdm496. [DOI] [PubMed] [Google Scholar]
- 128.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, Chau I, Van Cutsem E. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 129.Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, Canon JL, Van Laethem JL, Maurel J, Richardson G, Wolf M, Amado RG. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658–1664. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 130.Bardelli A, Siena S. Molecular mechanisms of resistance to cetuximab and panitumumab in colorectal cancer. J Clin Oncol. 2010;28:1254–1261. doi: 10.1200/JCO.2009.24.6116. [DOI] [PubMed] [Google Scholar]
- 131.Liao HW, Hsu JM, Xia W, Wang HL, Wang YN, Chang WC, Arold ST, Chou CK, Tsou PH, Yamaguchi H, Fang YF, Lee HJ, Lee HH, Tai SK, Yang MH, Morelli MP, Sen M, Ladbury JE, Chen CH, Grandis JR, Kopetz S, Hung MC. PRMT1-mediated methylation of the EGF receptor regulates signaling and cetuximab response. J Clin Invest. 2015;125:4529–4543. doi: 10.1172/JCI82826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bertotti A, Papp E, Jones S, Adleff V, Anagnostou V, Lupo B, Sausen M, Phallen J, Hruban CA, Tokheim C, Niknafs N, Nesselbush M, Lytle K, Sassi F, Cottino F, Migliardi G, Zanella ER, Ribero D, Russolillo N, Mellano A, Muratore A, Paraluppi G, Salizzoni M, Marsoni S, Kragh M, Lantto J, Cassingena A, Li QK, Karchin R, Scharpf R, Sartore-Bianchi A, Siena S, Diaz LA Jr, Trusolino L, Velculescu VE. The genomic landscape of response to EGFR blockade in colorectal cancer. Nature. 2015;526:263–267. doi: 10.1038/nature14969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Van Emburgh BO, Arena S, Siravegna G, Lazzari L, Crisafulli G, Corti G, Mussolin B, Baldi F, Buscarino M, Bartolini A, Valtorta E, Vidal J, Bellosillo B, Germano G, Pietrantonio F, Ponzetti A, Albanell J, Siena S, Sartore-Bianchi A, Di Nicolantonio F, Montagut C, Bardelli A. Acquired RAS or EGFR mutations and duration of response to EGFR blockade in colorectal cancer. Nat Commun. 2016;7:13665. doi: 10.1038/ncomms13665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Price T, Ang A, Boedigheimer M, Kim TW, Li J, Cascinu S, Ruff P, Satya Suresh A, Thomas A, Tjulandin S, Peeters M. Frequency of S492R mutations in the epidermal growth factor receptor: analysis of plasma DNA from patients with metastatic colorectal cancer treated with panitumumab or cetuximab monotherapy. Cancer Biol Ther. 2020;21:891–898. doi: 10.1080/15384047.2020.1798695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhou J, Ji Q, Li Q. Resistance to anti-EGFR therapies in metastatic colorectal cancer: underlying mechanisms and reversal strategies. J Exp Clin Cancer Res. 2021;40:328. doi: 10.1186/s13046-021-02130-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.De Roock W, Claes B, Bernasconi D, De Schutter J, Biesmans B, Fountzilas G, Kalogeras KT, Kotoula V, Papamichael D, Laurent-Puig P, Penault-Llorca F, Rougier P, Vincenzi B, Santini D, Tonini G, Cappuzzo F, Frattini M, Molinari F, Saletti P, De Dosso S, Martini M, Bardelli A, Siena S, Sartore-Bianchi A, Tabernero J, Macarulla T, Di Fiore F, Gangloff AO, Ciardiello F, Pfeiffer P, Qvortrup C, Hansen TP, Van Cutsem E, Piessevaux H, Lambrechts D, Delorenzi M, Tejpar S. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010;11:753–762. doi: 10.1016/S1470-2045(10)70130-3. [DOI] [PubMed] [Google Scholar]
- 137.Molinari F, Martin V, Saletti P, De Dosso S, Spitale A, Camponovo A, Bordoni A, Crippa S, Mazzucchelli L, Frattini M. Differing deregulation of EGFR and downstream proteins in primary colorectal cancer and related metastatic sites may be clinically relevant. Br J Cancer. 2009;100:1087–1094. doi: 10.1038/sj.bjc.6604848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Van Cutsem E, Köhne CH, Láng I, Folprecht G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D, Tejpar S, Schlichting M, Zubel A, Celik I, Rougier P, Ciardiello F. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011–2019. doi: 10.1200/JCO.2010.33.5091. [DOI] [PubMed] [Google Scholar]
- 139.Cremolini C, Rossini D, Dell'Aquila E, Lonardi S, Conca E, Del Re M, Busico A, Pietrantonio F, Danesi R, Aprile G, Tamburini E, Barone C, Masi G, Pantano F, Pucci F, Corsi DC, Pella N, Bergamo F, Rofi E, Barbara C, Falcone A, Santini D. Rechallenge for Patients With RAS and BRAF Wild-Type Metastatic Colorectal Cancer With Acquired Resistance to First-line Cetuximab and Irinotecan: A Phase 2 Single-Arm Clinical Trial. JAMA Oncol. 2019;5:343–350. doi: 10.1001/jamaoncol.2018.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ciardiello F, Normanno N, Maiello E, Martinelli E, Troiani T, Pisconti S, Giuliani F, Barone C, Cartenì G, Rachiglio AM, Montesarchio V, Tonini G, Rizzi D, Cinieri S, Bordonaro R, Febbraro A, De Vita F, Orditura M, Fenizia F, Lambiase M, Rinaldi A, Tatangelo F, Botti G, Colucci G. Clinical activity of FOLFIRI plus cetuximab according to extended gene mutation status by next-generation sequencing: findings from the CAPRI-GOIM trial. Ann Oncol. 2014;25:1756–1761. doi: 10.1093/annonc/mdu230. [DOI] [PubMed] [Google Scholar]
- 141.Perrone F, Lampis A, Orsenigo M, Di Bartolomeo M, Gevorgyan A, Losa M, Frattini M, Riva C, Andreola S, Bajetta E, Bertario L, Leo E, Pierotti MA, Pilotti S. PI3KCA/PTEN deregulation contributes to impaired responses to cetuximab in metastatic colorectal cancer patients. Ann Oncol. 2009;20:84–90. doi: 10.1093/annonc/mdn541. [DOI] [PubMed] [Google Scholar]
- 142.Jhawer M, Goel S, Wilson AJ, Montagna C, Ling YH, Byun DS, Nasser S, Arango D, Shin J, Klampfer L, Augenlicht LH, Perez-Soler R, Mariadason JM. PIK3CA mutation/PTEN expression status predicts response of colon cancer cells to the epidermal growth factor receptor inhibitor cetuximab. Cancer Res. 2008;68:1953–1961. doi: 10.1158/0008-5472.CAN-07-5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Han Y, Peng Y, Fu Y, Cai C, Guo C, Liu S, Li Y, Chen Y, Shen E, Long K, Wang X, Yu J, Shen H, Zeng S. MLH1 Deficiency Induces Cetuximab Resistance in Colon Cancer via Her-2/PI3K/AKT Signaling. Adv Sci (Weinh) 2020;7:2000112. doi: 10.1002/advs.202000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Luraghi P, Reato G, Cipriano E, Sassi F, Orzan F, Bigatto V, De Bacco F, Menietti E, Han M, Rideout WM 3rd, Perera T, Bertotti A, Trusolino L, Comoglio PM, Boccaccio C. MET signaling in colon cancer stem-like cells blunts the therapeutic response to EGFR inhibitors. Cancer Res. 2014;74:1857–1869. doi: 10.1158/0008-5472.CAN-13-2340-T. [DOI] [PubMed] [Google Scholar]
- 145.Lin SP, Lee YT, Yang SH, Miller SA, Chiou SH, Hung MC, Hung SC. Colon cancer stem cells resist antiangiogenesis therapy-induced apoptosis. Cancer Lett. 2013;328:226–234. doi: 10.1016/j.canlet.2012.08.036. [DOI] [PubMed] [Google Scholar]
- 146.Becherirat S, Valamanesh F, Karimi M, Faussat AM, Launay JM, Pimpie C, Therwath A, Pocard M. Discontinuous Schedule of Bevacizumab in Colorectal Cancer Induces Accelerated Tumor Growth and Phenotypic Changes. Transl Oncol. 2018;11:406–415. doi: 10.1016/j.tranon.2018.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Maddalena F, Condelli V, Matassa DS, Pacelli C, Scrima R, Lettini G, Li Bergolis V, Pietrafesa M, Crispo F, Piscazzi A, Storto G, Capitanio N, Esposito F, Landriscina M. TRAP1 enhances Warburg metabolism through modulation of PFK1 expression/activity and favors resistance to EGFR inhibitors in human colorectal carcinomas. Mol Oncol. 2020;14:3030–3047. doi: 10.1002/1878-0261.12814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Skvortsov S, Sarg B, Loeffler-Ragg J, Skvortsova I, Lindner H, Werner Ott H, Lukas P, Illmensee K, Zwierzina H. Different proteome pattern of epidermal growth factor receptor-positive colorectal cancer cell lines that are responsive and nonresponsive to C225 antibody treatment. Mol Cancer Ther. 2004;3:1551–1558. [PubMed] [Google Scholar]
- 149.Sidaway P. Microenvironment plasticity confers cetuximab resistance. Nat Rev Clin Oncol. 2019;16:527. doi: 10.1038/s41571-019-0259-4. [DOI] [PubMed] [Google Scholar]
- 150.De Angelis ML, Zeuner A, Policicchio E, Russo G, Bruselles A, Signore M, Vitale S, De Luca G, Pilozzi E, Boe A, Stassi G, Ricci-Vitiani L, Amoreo CA, Pagliuca A, Francescangeli F, Tartaglia M, De Maria R, Baiocchi M. Cancer Stem Cell-Based Models of Colorectal Cancer Reveal Molecular Determinants of Therapy Resistance. Stem Cells Transl Med. 2016;5:511–523. doi: 10.5966/sctm.2015-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Calemma R, Ottaiano A, Trotta AM, Nasti G, Romano C, Napolitano M, Galati D, Borrelli P, Zanotta S, Cassata A, Castello G, Iaffaioli VR, Scala S. Fc gamma receptor IIIa polymorphisms in advanced colorectal cancer patients correlated with response to anti-EGFR antibodies and clinical outcome. J Transl Med. 2012;10:232. doi: 10.1186/1479-5876-10-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Woolston A, Khan K, Spain G, Barber LJ, Griffiths B, Gonzalez-Exposito R, Hornsteiner L, Punta M, Patil Y, Newey A, Mansukhani S, Davies MN, Furness A, Sclafani F, Peckitt C, Jiménez M, Kouvelakis K, Ranftl R, Begum R, Rana I, Thomas J, Bryant A, Quezada S, Wotherspoon A, Khan N, Fotiadis N, Marafioti T, Powles T, Lise S, Calvo F, Guettler S, von Loga K, Rao S, Watkins D, Starling N, Chau I, Sadanandam A, Cunningham D, Gerlinger M. Genomic and Transcriptomic Determinants of Therapy Resistance and Immune Landscape Evolution during Anti-EGFR Treatment in Colorectal Cancer. Cancer Cell. 2019;36:35–50.e9. doi: 10.1016/j.ccell.2019.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Walcher L, Kistenmacher AK, Suo H, Kitte R, Dluczek S, Strauß A, Blaudszun AR, Yevsa T, Fricke S, Kossatz-Boehlert U. Cancer Stem Cells-Origins and Biomarkers: Perspectives for Targeted Personalized Therapies. Front Immunol. 2020;11:1280. doi: 10.3389/fimmu.2020.01280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang T, Song P, Zhong T, Wang X, Xiang X, Liu Q, Chen H, Xia T, Liu H, Niu Y, Hu Y, Xu L, Shao Y, Zhu L, Qi H, Shen J, Hou T, Fodde R, Shao J. The inflammatory cytokine IL-6 induces FRA1 deacetylation promoting colorectal cancer stem-like properties. Oncogene. 2019;38:4932–4947. doi: 10.1038/s41388-019-0763-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, Adenis A, Tabernero J, Yoshino T, Lenz HJ, Goldberg RM, Sargent DJ, Cihon F, Cupit L, Wagner A, Laurent D CORRECT Study Group. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 156.Tabernero J, Yoshino T, Cohn AL, Obermannova R, Bodoky G, Garcia-Carbonero R, Ciuleanu TE, Portnoy DC, Van Cutsem E, Grothey A, Prausová J, Garcia-Alfonso P, Yamazaki K, Clingan PR, Lonardi S, Kim TW, Simms L, Chang SC, Nasroulah F RAISE Study Investigators. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015;16:499–508. doi: 10.1016/S1470-2045(15)70127-0. [DOI] [PubMed] [Google Scholar]
- 157.Van Cutsem E, Tabernero J, Lakomy R, Prenen H, Prausová J, Macarulla T, Ruff P, van Hazel GA, Moiseyenko V, Ferry D, McKendrick J, Polikoff J, Tellier A, Castan R, Allegra C. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30:3499–3506. doi: 10.1200/JCO.2012.42.8201. [DOI] [PubMed] [Google Scholar]
- 158.Ellis LM, Hicklin DJ. Pathways mediating resistance to vascular endothelial growth factor-targeted therapy. Clin Cancer Res. 2008;14:6371–6375. doi: 10.1158/1078-0432.CCR-07-5287. [DOI] [PubMed] [Google Scholar]
- 159.Codony-Servat J, Cuatrecasas M, Asensio E, Montironi C, Martínez-Cardús A, Marín-Aguilera M, Horndler C, Martínez-Balibrea E, Rubini M, Jares P, Reig O, Victoria I, Gaba L, Martín-Richard M, Alonso V, Escudero P, Fernández-Martos C, Feliu J, Méndez JC, Méndez M, Gallego J, Salud A, Rojo F, Castells A, Prat A, Rosell R, García-Albéniz X, Camps J, Maurel J. Nuclear IGF-1R predicts chemotherapy and targeted therapy resistance in metastatic colorectal cancer. Br J Cancer. 2017;117:1777–1786. doi: 10.1038/bjc.2017.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Clarke PA, Roe T, Swabey K, Hobbs SM, McAndrew C, Tomlin K, Westwood I, Burke R, van Montfort R, Workman P. Dissecting mechanisms of resistance to targeted drug combination therapy in human colorectal cancer. Oncogene. 2019;38:5076–5090. doi: 10.1038/s41388-019-0780-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Itatani Y, Kawada K, Yamamoto T, Sakai Y. Resistance to Anti-Angiogenic Therapy in Cancer-Alterations to Anti-VEGF Pathway. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19041232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Jayson GC, Kerbel R, Ellis LM, Harris AL. Antiangiogenic therapy in oncology: current status and future directions. Lancet. 2016;388:518–529. doi: 10.1016/S0140-6736(15)01088-0. [DOI] [PubMed] [Google Scholar]
- 163.Itatani Y, Yamamoto T, Zhong C, Molinolo AA, Ruppel J, Hegde P, Taketo MM, Ferrara N. Suppressing neutrophil-dependent angiogenesis abrogates resistance to anti-VEGF antibody in a genetic model of colorectal cancer. Proc Natl Acad Sci U S A. 2020;117:21598–21608. doi: 10.1073/pnas.2008112117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Schiffmann LM, Fritsch M, Gebauer F, Günther SD, Stair NR, Seeger JM, Thangarajah F, Dieplinger G, Bludau M, Alakus H, Göbel H, Quaas A, Zander T, Hilberg F, Bruns CJ, Kashkar H, Coutelle O. Tumour-infiltrating neutrophils counteract anti-VEGF therapy in metastatic colorectal cancer. Br J Cancer. 2019;120:69–78. doi: 10.1038/s41416-018-0198-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Carbone C, Piro G, Simionato F, Ligorio F, Cremolini C, Loupakis F, Alì G, Rossini D, Merz V, Santoro R, Zecchetto C, Zanotto M, Di Nicolantonio F, Bardelli A, Fontanini G, Tortora G, Melisi D. Homeobox B9 Mediates Resistance to Anti-VEGF Therapy in Colorectal Cancer Patients. Clin Cancer Res. 2017;23:4312–4322. doi: 10.1158/1078-0432.CCR-16-3153. [DOI] [PubMed] [Google Scholar]
- 166.Rahbari NN, Kedrin D, Incio J, Liu H, Ho WW, Nia HT, Edrich CM, Jung K, Daubriac J, Chen I, Heishi T, Martin JD, Huang Y, Maimon N, Reissfelder C, Weitz J, Boucher Y, Clark JW, Grodzinsky AJ, Duda DG, Jain RK, Fukumura D. Anti-VEGF therapy induces ECM remodeling and mechanical barriers to therapy in colorectal cancer liver metastases. Sci Transl Med. 2016;8:360ra135. doi: 10.1126/scitranslmed.aaf5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Park YL, Kim HP, Ock CY, Min DW, Kang JK, Lim YJ, Song SH, Han SW, Kim TY. EMT-mediated regulation of CXCL1/5 for resistance to anti-EGFR therapy in colorectal cancer. Oncogene. 2022;41:2026–2038. doi: 10.1038/s41388-021-01920-4. [DOI] [PubMed] [Google Scholar]
- 168.Holch JW, Demmer M, Lamersdorf C, Michl M, Schulz C, von Einem JC, Modest DP, Heinemann V. Pattern and Dynamics of Distant Metastases in Metastatic Colorectal Cancer. Visc Med. 2017;33:70–75. doi: 10.1159/000454687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Schmoll HJ, Van Cutsem E, Stein A, Valentini V, Glimelius B, Haustermans K, Nordlinger B, van de Velde CJ, Balmana J, Regula J, Nagtegaal ID, Beets-Tan RG, Arnold D, Ciardiello F, Hoff P, Kerr D, Köhne CH, Labianca R, Price T, Scheithauer W, Sobrero A, Tabernero J, Aderka D, Barroso S, Bodoky G, Douillard JY, El Ghazaly H, Gallardo J, Garin A, Glynne-Jones R, Jordan K, Meshcheryakov A, Papamichail D, Pfeiffer P, Souglakos I, Turhal S, Cervantes A. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann Oncol. 2012;23:2479–2516. doi: 10.1093/annonc/mds236. [DOI] [PubMed] [Google Scholar]
- 170.Böckelman C, Engelmann BE, Kaprio T, Hansen TF, Glimelius B. Risk of recurrence in patients with colon cancer stage II and III: a systematic review and meta-analysis of recent literature. Acta Oncol. 2015;54:5–16. doi: 10.3109/0284186X.2014.975839. [DOI] [PubMed] [Google Scholar]
- 171.Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8:98–101. [PubMed] [Google Scholar]
- 172.Zhou H, Liu Z, Wang Y, Wen X, Amador EH, Yuan L, Ran X, Xiong L, Ran Y, Chen W, Wen Y. Colorectal liver metastasis: molecular mechanism and interventional therapy. Signal Transduct Target Ther. 2022;7:70. doi: 10.1038/s41392-022-00922-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tauriello DV, Calon A, Lonardo E, Batlle E. Determinants of metastatic competency in colorectal cancer. Mol Oncol. 2017;11:97–119. doi: 10.1002/1878-0261.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Khan U, Chowdhury S, Billah MM, Islam KMD, Thorlacius H, Rahman M. Neutrophil Extracellular Traps in Colorectal Cancer Progression and Metastasis. Int J Mol Sci. 2021;22 doi: 10.3390/ijms22147260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lugli A, Zlobec I, Berger MD, Kirsch R, Nagtegaal ID. Tumour budding in solid cancers. Nat Rev Clin Oncol. 2021;18:101–115. doi: 10.1038/s41571-020-0422-y. [DOI] [PubMed] [Google Scholar]
- 176.Jinesh GG, Brohl AS. Classical epithelial-mesenchymal transition (EMT) and alternative cell death process-driven blebbishield metastatic-witch (BMW) pathways to cancer metastasis. Signal Transduct Target Ther. 2022;7:296. doi: 10.1038/s41392-022-01132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Enquist IB, Good Z, Jubb AM, Fuh G, Wang X, Junttila MR, Jackson EL, Leong KG. Lymph node-independent liver metastasis in a model of metastatic colorectal cancer. Nat Commun. 2014;5:3530. doi: 10.1038/ncomms4530. [DOI] [PubMed] [Google Scholar]
- 178.Leake I. Colorectal cancer. Understanding the routes of metastasis in colorectal cancer. Nat Rev Gastroenterol Hepatol. 2014;11:270. doi: 10.1038/nrgastro.2014.60. [DOI] [PubMed] [Google Scholar]
- 179.Tauriello DVF, Palomo-Ponce S, Stork D, Berenguer-Llergo A, Badia-Ramentol J, Iglesias M, Sevillano M, Ibiza S, Cañellas A, Hernando-Momblona X, Byrom D, Matarin JA, Calon A, Rivas EI, Nebreda AR, Riera A, Attolini CS, Batlle E. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature. 2018;554:538–543. doi: 10.1038/nature25492. [DOI] [PubMed] [Google Scholar]
- 180.Ji Q, Liu X, Han Z, Zhou L, Sui H, Yan L, Jiang H, Ren J, Cai J, Li Q. Resveratrol suppresses epithelial-to-mesenchymal transition in colorectal cancer through TGF-β1/Smads signaling pathway mediated Snail/E-cadherin expression. BMC Cancer. 2015;15:97. doi: 10.1186/s12885-015-1119-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Jinesh GG, Brohl AS. The genetic script of metastasis. Biol Rev Camb Philos Soc. 2020;95:244–266. doi: 10.1111/brv.12562. [DOI] [PubMed] [Google Scholar]
- 182.Jinesh GG, Kamat AM. Blebbishield emergency program: an apoptotic route to cellular transformation. Cell Death Differ. 2016;23:757–758. doi: 10.1038/cdd.2016.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Jinesh GG, Kamat AM. The Blebbishield Emergency Program Overrides Chromosomal Instability and Phagocytosis Checkpoints in Cancer Stem Cells. Cancer Res. 2017;77:6144–6156. doi: 10.1158/0008-5472.CAN-17-0522. [DOI] [PubMed] [Google Scholar]
- 184.Jinesh GG, Choi W, Shah JB, Lee EK, Willis DL, Kamat AM. Blebbishields, the emergency program for cancer stem cells: sphere formation and tumorigenesis after apoptosis. Cell Death Differ. 2013;20:382–395. doi: 10.1038/cdd.2012.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tang HM, Tang HL. Cell recovery by reversal of ferroptosis. Biol Open. 2019;8 doi: 10.1242/bio.043182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bergers G, Fendt SM. The metabolism of cancer cells during metastasis. Nat Rev Cancer. 2021;21:162–180. doi: 10.1038/s41568-020-00320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Shelton LM, Huysentruyt LC, Seyfried TN. Glutamine targeting inhibits systemic metastasis in the VM-M3 murine tumor model. Int J Cancer. 2010;127:2478–2485. doi: 10.1002/ijc.25431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Xiang L, Mou J, Shao B, Wei Y, Liang H, Takano N, Semenza GL, Xie G. Glutaminase 1 expression in colorectal cancer cells is induced by hypoxia and required for tumor growth, invasion, and metastatic colonization. Cell Death Dis. 2019;10:40. doi: 10.1038/s41419-018-1291-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wang YN, Zeng ZL, Lu J, Wang Y, Liu ZX, He MM, Zhao Q, Wang ZX, Li T, Lu YX, Wu QN, Yu K, Wang F, Pu HY, Li B, Jia WH, Shi M, Xie D, Kang TB, Huang P, Ju HQ, Xu RH. CPT1A-mediated fatty acid oxidation promotes colorectal cancer cell metastasis by inhibiting anoikis. Oncogene. 2018;37:6025–6040. doi: 10.1038/s41388-018-0384-z. [DOI] [PubMed] [Google Scholar]
- 190.Kaplan MJ, Radic M. Neutrophil extracellular traps: double-edged swords of innate immunity. J Immunol. 2012;189:2689–2695. doi: 10.4049/jimmunol.1201719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Xing X, Bai Y, Song J. The Heterogeneity of Neutrophil Recruitment in the Tumor Microenvironment and the Formation of Premetastatic Niches. J Immunol Res. 2021;2021:6687474. doi: 10.1155/2021/6687474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kowanetz M, Wu X, Lee J, Tan M, Hagenbeek T, Qu X, Yu L, Ross J, Korsisaari N, Cao T, Bou-Reslan H, Kallop D, Weimer R, Ludlam MJ, Kaminker JS, Modrusan Z, van Bruggen N, Peale FV, Carano R, Meng YG, Ferrara N. Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc Natl Acad Sci U S A. 2010;107:21248–21255. doi: 10.1073/pnas.1015855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Arelaki S, Arampatzioglou A, Kambas K, Papagoras C, Miltiades P, Angelidou I, Mitsios A, Kotsianidis I, Skendros P, Sivridis E, Maroulakou I, Giatromanolaki A, Ritis K. Gradient Infiltration of Neutrophil Extracellular Traps in Colon Cancer and Evidence for Their Involvement in Tumour Growth. PLoS One. 2016;11:e0154484. doi: 10.1371/journal.pone.0154484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Tohme S, Yazdani HO, Al-Khafaji AB, Chidi AP, Loughran P, Mowen K, Wang Y, Simmons RL, Huang H, Tsung A. Neutrophil Extracellular Traps Promote the Development and Progression of Liver Metastases after Surgical Stress. Cancer Res. 2016;76:1367–1380. doi: 10.1158/0008-5472.CAN-15-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Richardson JJR, Hendrickse C, Gao-Smith F, Thickett DR. Neutrophil Extracellular Trap Production in Patients with Colorectal Cancer In vitro. Int J Inflam. 2017;2017:4915062. doi: 10.1155/2017/4915062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Shang A, Gu C, Zhou C, Yang Y, Chen C, Zeng B, Wu J, Lu W, Wang W, Sun Z, Li D. Exosomal KRAS mutation promotes the formation of tumor-associated neutrophil extracellular traps and causes deterioration of colorectal cancer by inducing IL-8 expression. Cell Commun Signal. 2020;18:52. doi: 10.1186/s12964-020-0517-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Carroll GM, Burns GL, Petit JA, Walker MM, Mathe A, Smith SR, Keely S, Pockney PG. Does postoperative inflammation or sepsis generate neutrophil extracellular traps that influence colorectal cancer progression? Surg Open Sci. 2020;2:57–69. doi: 10.1016/j.sopen.2019.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Walther A, Johnstone E, Swanton C, Midgley R, Tomlinson I, Kerr D. Genetic prognostic and predictive markers in colorectal cancer. Nat Rev Cancer. 2009;9:489–499. doi: 10.1038/nrc2645. [DOI] [PubMed] [Google Scholar]
- 199.Yang L, Liu L, Zhang R, Hong J, Wang Y, Wang J, Zuo J, Zhang J, Chen J, Hao H. IL-8 mediates a positive loop connecting increased neutrophil extracellular traps (NETs) and colorectal cancer liver metastasis. J Cancer. 2020;11:4384–4396. doi: 10.7150/jca.44215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Rayes RF, Mouhanna JG, Nicolau I, Bourdeau F, Giannias B, Rousseau S, Quail D, Walsh L, Sangwan V, Bertos N, Cools-Lartigue J, Ferri LE, Spicer JD. Primary tumors induce neutrophil extracellular traps with targetable metastasis promoting effects. JCI Insight. 2019;5 doi: 10.1172/jci.insight.128008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Mizuno R, Kawada K, Itatani Y, Ogawa R, Kiyasu Y, Sakai Y. The Role of Tumor-Associated Neutrophils in Colorectal Cancer. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20030529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Yazdani HO, Roy E, Comerci AJ, van der Windt DJ, Zhang H, Huang H, Loughran P, Shiva S, Geller DA, Bartlett DL, Tsung A, Sheng T, Simmons RL, Tohme S. Neutrophil Extracellular Traps Drive Mitochondrial Homeostasis in Tumors to Augment Growth. Cancer Res. 2019;79:5626–5639. doi: 10.1158/0008-5472.CAN-19-0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Rayes RF, Vourtzoumis P, Bou Rjeily M, Seth R, Bourdeau F, Giannias B, Berube J, Huang YH, Rousseau S, Camilleri-Broet S, Blumberg RS, Beauchemin N, Najmeh S, Cools-Lartigue J, Spicer JD, Ferri LE. Neutrophil Extracellular Trap-Associated CEACAM1 as a Putative Therapeutic Target to Prevent Metastatic Progression of Colon Carcinoma. J Immunol. 2020;204:2285–2294. doi: 10.4049/jimmunol.1900240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Xia Y, He J, Zhang H, Wang H, Tetz G, Maguire CA, Wang Y, Onuma A, Genkin D, Tetz V, Stepanov A, Terekhov S, Ukrainskaya V, Huang H, Tsung A. AAV-mediated gene transfer of DNase I in the liver of mice with colorectal cancer reduces liver metastasis and restores local innate and adaptive immune response. Mol Oncol. 2020;14:2920–2935. doi: 10.1002/1878-0261.12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wei C, Yang C, Wang S, Shi D, Zhang C, Lin X, Liu Q, Dou R, Xiong B. Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis. Mol Cancer. 2019;18:64. doi: 10.1186/s12943-019-0976-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Pavitra E, Dariya B, Srivani G, Kang SM, Alam A, Sudhir PR, Kamal MA, Raju GSR, Han YK, Lakkakula BVKS, Nagaraju GP, Huh YS. Engineered nanoparticles for imaging and drug delivery in colorectal cancer. Semin Cancer Biol. 2021;69:293–306. doi: 10.1016/j.semcancer.2019.06.017. [DOI] [PubMed] [Google Scholar]
- 207.Yang B, Chen Y, Shi J. Exosome Biochemistry and Advanced Nanotechnology for Next-Generation Theranostic Platforms. Adv Mater. 2019;31:e1802896. doi: 10.1002/adma.201802896. [DOI] [PubMed] [Google Scholar]
- 208.Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, Klyachko NL, Inskoe E, Piroyan A, Sokolsky M, Okolie O, Hingtgen SD, Kabanov AV, Batrakova EV. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine. 2016;12:655–664. doi: 10.1016/j.nano.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Abello J, Nguyen TDT, Marasini R, Aryal S, Weiss ML. Biodistribution of gadolinium- and near infrared-labeled human umbilical cord mesenchymal stromal cell-derived exosomes in tumor bearing mice. Theranostics. 2019;9:2325–2345. doi: 10.7150/thno.30030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ren J, He W, Zheng L, Duan H. From structures to functions: insights into exosomes as promising drug delivery vehicles. Biomater Sci. 2016;4:910–921. doi: 10.1039/c5bm00583c. [DOI] [PubMed] [Google Scholar]
- 211.Li H, Yao Q, Sun W, Shao K, Lu Y, Chung J, Kim D, Fan J, Long S, Du J, Li Y, Wang J, Yoon J, Peng X. Aminopeptidase N Activatable Fluorescent Probe for Tracking Metastatic Cancer and Image-Guided Surgery via in Situ Spraying. J Am Chem Soc. 2020;142:6381–6389. doi: 10.1021/jacs.0c01365. [DOI] [PubMed] [Google Scholar]
- 212.Wang J, Zhang L, Su Y, Qu Y, Cao Y, Qin W, Liu Y. A Novel Fluorescent Probe Strategy Activated by β-Glucuronidase for Assisting Surgical Resection of Liver Cancer. Anal Chem. 2022;94:7012–7020. doi: 10.1021/acs.analchem.1c05635. [DOI] [PubMed] [Google Scholar]
- 213.Correa S, Dreaden EC, Gu L, Hammond PT. Engineering nanolayered particles for modular drug delivery. J Control Release. 2016;240:364–386. doi: 10.1016/j.jconrel.2016.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Hu Y, Zhang K, Zhu X, Zheng X, Wang C, Niu X, Jiang T, Ji X, Zhao W, Pang L, Qi Y, Li F, Li L, Xu Z, Gu W, Zou H. Synergistic Inhibition of Drug-Resistant Colon Cancer Growth with PI3K/mTOR Dual Inhibitor BEZ235 and Nano-Emulsioned Paclitaxel via Reducing Multidrug Resistance and Promoting Apoptosis. Int J Nanomedicine. 2021;16:2173–2186. doi: 10.2147/IJN.S290731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kannan P, Kretzschmar WW, Winter H, Warren D, Bates R, Allen PD, Syed N, Irving B, Papiez BW, Kaeppler J, Markelc B, Kinchesh P, Gilchrist S, Smart S, Schnabel JA, Maughan T, Harris AL, Muschel RJ, Partridge M, Sharma RA, Kersemans V. Functional Parameters Derived from Magnetic Resonance Imaging Reflect Vascular Morphology in Preclinical Tumors and in Human Liver Metastases. Clin Cancer Res. 2018;24:4694–4704. doi: 10.1158/1078-0432.CCR-18-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Feng ST, Li J, Luo Y, Yin T, Cai H, Wang Y, Dong Z, Shuai X, Li ZP. pH-sensitive nanomicelles for controlled and efficient drug delivery to human colorectal carcinoma LoVo cells. PLoS One. 2014;9:e100732. doi: 10.1371/journal.pone.0100732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Boissenot T, Fattal E, Bordat A, Houvenagel S, Valette J, Chacun H, Gueutin C, Tsapis N. Paclitaxel-loaded PEGylated nanocapsules of perfluorooctyl bromide as theranostic agents. Eur J Pharm Biopharm. 2016;108:136–144. doi: 10.1016/j.ejpb.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 218.Shakeri-Zadeh A, Shiran MB, Khoee S, Sharifi AM, Ghaznavi H, Khoei S. A new magnetic nanocapsule containing 5-fluorouracil: in vivo drug release, anti-tumor, and pro-apoptotic effects on CT26 cells allograft model. J Biomater Appl. 2014;29:548–556. doi: 10.1177/0885328214536940. [DOI] [PubMed] [Google Scholar]
- 219.Voulgari E, Bakandritsos A, Galtsidis S, Zoumpourlis V, Burke BP, Clemente GS, Cawthorne C, Archibald SJ, Tuček J, Zbořil R, Kantarelou V, Karydas AG, Avgoustakis K. Synthesis, characterization and in vivo evaluation of a magnetic cisplatin delivery nanosystem based on PMAA-graft-PEG copolymers. J Control Release. 2016;243:342–356. doi: 10.1016/j.jconrel.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 220.Li LH, Dong H, Zhao F, Tang J, Chen X, Ding J, Men HT, Luo WX, Du Y, Ge J, Tan BX, Cao D, Liu JY. The upregulation of dihydropyrimidine dehydrogenase in liver is involved in acquired resistance to 5-fluorouracil. Eur J Cancer. 2013;49:1752–1760. doi: 10.1016/j.ejca.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 221.Negrei C, Hudita A, Ginghina O, Galateanu B, Voicu SN, Stan M, Costache M, Fenga C, Drakoulis N, Tsatsakis AM. Colon Cancer Cells Gene Expression Signature As Response to 5- Fluorouracil, Oxaliplatin, and Folinic Acid Treatment. Front Pharmacol. 2016;7:172. doi: 10.3389/fphar.2016.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Entezar-Almahdi E, Mohammadi-Samani S, Tayebi L, Farjadian F. Recent Advances in Designing 5-Fluorouracil Delivery Systems: A Stepping Stone in the Safe Treatment of Colorectal Cancer. Int J Nanomedicine. 2020;15:5445–5458. doi: 10.2147/IJN.S257700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.González-Lavado E, Valdivia L, García-Castaño A, González F, Pesquera C, Valiente R, Fanarraga ML. Multi-walled carbon nanotubes complement the anti-tumoral effect of 5-Fluorouracil. Oncotarget. 2019;10:2022–2029. doi: 10.18632/oncotarget.26770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Udofot O, Affram K, Smith T, Tshabe B, Krishnan S, Sachdeva M, Agyare E. Pharmacokinetic, biodistribution and therapeutic efficacy of 5-fluorouracil-loaded pH-sensitive PEGylated liposomal nanoparticles in HCT-116 tumor bearing mouse. J Nat Sci. 2016;2 [PMC free article] [PubMed] [Google Scholar]
- 225.Safwat MA, Soliman GM, Sayed D, Attia MA. Gold nanoparticles enhance 5-fluorouracil anticancer efficacy against colorectal cancer cells. Int J Pharm. 2016;513:648–658. doi: 10.1016/j.ijpharm.2016.09.076. [DOI] [PubMed] [Google Scholar]
- 226.Russo A, Maiolino S, Pagliara V, Ungaro F, Tatangelo F, Leone A, Scalia G, Budillon A, Quaglia F, Russo G. Enhancement of 5-FU sensitivity by the proapoptotic rpL3 gene in p53 null colon cancer cells through combined polymer nanoparticles. Oncotarget. 2016;7:79670–79687. doi: 10.18632/oncotarget.13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Guilford JM, Pezzuto JM. Natural products as inhibitors of carcinogenesis. Expert Opin Investig Drugs. 2008;17:1341–1352. doi: 10.1517/13543784.17.9.1341. [DOI] [PubMed] [Google Scholar]
- 228.Li L, Ahmed B, Mehta K, Kurzrock R. Liposomal curcumin with and without oxaliplatin: effects on cell growth, apoptosis, and angiogenesis in colorectal cancer. Mol Cancer Ther. 2007;6:1276–1282. doi: 10.1158/1535-7163.MCT-06-0556. [DOI] [PubMed] [Google Scholar]
- 229.Tefas LR, Sylvester B, Tomuta I, Sesarman A, Licarete E, Banciu M, Porfire A. Development of antiproliferative long-circulating liposomes co-encapsulating doxorubicin and curcumin, through the use of a quality-by-design approach. Drug Des Devel Ther. 2017;11:1605–1621. doi: 10.2147/DDDT.S129008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Raveendran R, Bhuvaneshwar G, Sharma CP. In vitro cytotoxicity and cellular uptake of curcumin-loaded Pluronic/Polycaprolactone micelles in colorectal adenocarcinoma cells. J Biomater Appl. 2013;27:811–827. doi: 10.1177/0885328211427473. [DOI] [PubMed] [Google Scholar]
- 231.San Hipólito-Luengo Á, Alcaide A, Ramos-González M, Cercas E, Vallejo S, Romero A, Talero E, Sánchez-Ferrer CF, Motilva V, Peiró C. Dual Effects of Resveratrol on Cell Death and Proliferation of Colon Cancer Cells. Nutr Cancer. 2017;69:1019–1027. doi: 10.1080/01635581.2017.1359309. [DOI] [PubMed] [Google Scholar]
- 232.Kamal R, Chadha VD, Dhawan DK. Physiological uptake and retention of radiolabeled resveratrol loaded gold nanoparticles ((99m)Tc-Res-AuNP) in colon cancer tissue. Nanomedicine. 2018;14:1059–1071. doi: 10.1016/j.nano.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 233.Calviello G, Di Nicuolo F, Serini S, Piccioni E, Boninsegna A, Maggiano N, Ranelletti FO, Palozza P. Docosahexaenoic acid enhances the susceptibility of human colorectal cancer cells to 5-fluorouracil. Cancer Chemother Pharmacol. 2005;55:12–20. doi: 10.1007/s00280-004-0846-6. [DOI] [PubMed] [Google Scholar]
- 234.Serini S, Ottes Vasconcelos R, Fasano E, Calviello G. How plausible is the use of dietary n-3 PUFA in the adjuvant therapy of cancer? Nutr Res Rev. 2016;29:102–125. doi: 10.1017/S0954422416000044. [DOI] [PubMed] [Google Scholar]
- 235.Santandreu FM, Valle A, Oliver J, Roca P. Resveratrol potentiates the cytotoxic oxidative stress induced by chemotherapy in human colon cancer cells. Cell Physiol Biochem. 2011;28:219–228. doi: 10.1159/000331733. [DOI] [PubMed] [Google Scholar]
- 236.Mohapatra P, Preet R, Choudhuri M, Choudhuri T, Kundu CN. 5-fluorouracil increases the chemopreventive potentials of resveratrol through DNA damage and MAPK signaling pathway in human colorectal cancer cells. Oncol Res. 2011;19:311–321. doi: 10.3727/096504011x13079697132844. [DOI] [PubMed] [Google Scholar]
- 237.Hui B, Lu C, Wang J, Xu Y, Yang Y, Ji H, Li X, Xu L, Tang W, Wang K, Gu Y. Engineered exosomes for co-delivery of PGM5-AS1 and oxaliplatin to reverse drug resistance in colon cancer. J Cell Physiol. 2022;237:911–933. doi: 10.1002/jcp.30566. [DOI] [PubMed] [Google Scholar]
- 238.Tran PHL, Wang T, Yin W, Tran TTD, Barua HT, Zhang Y, Midge SB, Nguyen TNG, Lee BJ, Duan W. Development of a nanoamorphous exosomal delivery system as an effective biological platform for improved encapsulation of hydrophobic drugs. Int J Pharm. 2019;566:697–707. doi: 10.1016/j.ijpharm.2019.06.028. [DOI] [PubMed] [Google Scholar]
- 239.Darwis NDM, Nachankar A, Sasaki Y, Matsui T, Noda SE, Murata K, Tamaki T, Ando K, Okonogi N, Shiba S, Irie D, Kaminuma T, Kumazawa T, Anakura M, Yamashita S, Hirakawa T, Kakoti S, Hirota Y, Tokino T, Iwase A, Ohno T, Shibata A, Oike T, Nakano T. FGFR Signaling as a Candidate Therapeutic Target for Cancers Resistant to Carbon Ion Radiotherapy. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20184563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Matsui T, Nuryadi E, Komatsu S, Hirota Y, Shibata A, Oike T, Nakano T. Robustness of Clonogenic Assays as a Biomarker for Cancer Cell Radiosensitivity. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20174148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Sundaram A, Peng L, Chai L, Xie Z, Ponraj JS, Wang X, Wang G, Zhang B, Nie G, Xie N, Rajesh Kumar M, Zhang H. Advanced nanomaterials for hypoxia tumor therapy: challenges and solutions. Nanoscale. 2020;12:21497–21518. doi: 10.1039/d0nr06271e. [DOI] [PubMed] [Google Scholar]
- 242.Song G, Cheng L, Chao Y, Yang K, Liu Z. Emerging Nanotechnology and Advanced Materials for Cancer Radiation Therapy. Adv Mater. 2017;29 doi: 10.1002/adma.201700996. [DOI] [PubMed] [Google Scholar]
- 243.Jin Z, Zhao Q, Yuan S, Jiang W, Hu Y. Strategies of Alleviating Tumor Hypoxia and Enhancing Tumor Therapeutic Effect by Macromolecular Nanomaterials. Macromol Biosci. 2021;21:e2100092. doi: 10.1002/mabi.202100092. [DOI] [PubMed] [Google Scholar]
- 244.Chen Q, Feng L, Liu J, Zhu W, Dong Z, Wu Y, Liu Z. Intelligent Albumin-MnO2 Nanoparticles as pH-/H2 O2 -Responsive Dissociable Nanocarriers to Modulate Tumor Hypoxia for Effective Combination Therapy. Adv Mater. 2016;28:7129–7136. doi: 10.1002/adma.201601902. [DOI] [PubMed] [Google Scholar]
- 245.Tang W, Yang Z, He L, Deng L, Fathi P, Zhu S, Li L, Shen B, Wang Z, Jacobson O, Song J, Zou J, Hu P, Wang M, Mu J, Cheng Y, Ma Y, Tang L, Fan W, Chen X. A hybrid semiconducting organosilica-based O(2) nanoeconomizer for on-demand synergistic photothermally boosted radiotherapy. Nat Commun. 2021;12:523. doi: 10.1038/s41467-020-20860-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Her S, Jaffray DA, Allen C. Gold nanoparticles for applications in cancer radiotherapy: Mechanisms and recent advancements. Adv Drug Deliv Rev. 2017;109:84–101. doi: 10.1016/j.addr.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 247.Choi J, Kim G, Cho SB, Im HJ. Radiosensitizing high-Z metal nanoparticles for enhanced radiotherapy of glioblastoma multiforme. J Nanobiotechnology. 2020;18:122. doi: 10.1186/s12951-020-00684-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Penninckx S, Heuskin AC, Michiels C, Lucas S. Gold Nanoparticles as a Potent Radiosensitizer: A Transdisciplinary Approach from Physics to Patient. Cancers (Basel) 2020;12 doi: 10.3390/cancers12082021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Bromma K, Cicon L, Beckham W, Chithrani DB. Gold nanoparticle mediated radiation response among key cell components of the tumour microenvironment for the advancement of cancer nanotechnology. Sci Rep. 2020;10:12096. doi: 10.1038/s41598-020-68994-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Wang X, Zhang Y, Mu X, Tu CR, Chung Y, Tsao SW, Chan GC, Leung WH, Lau YL, Liu Y, Tu W. Exosomes derived from γδ-T cells synergize with radiotherapy and preserve antitumor activities against nasopharyngeal carcinoma in immunosuppressive microenvironment. J Immunother Cancer. 2022;10 doi: 10.1136/jitc-2021-003832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Wan FZ, Chen KH, Sun YC, Chen XC, Liang RB, Chen L, Zhu XD. Exosomes overexpressing miR-34c inhibit malignant behavior and reverse the radioresistance of nasopharyngeal carcinoma. J Transl Med. 2020;18:12. doi: 10.1186/s12967-019-02203-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Wang D, Gao G. State-of-the-art human gene therapy: part II. Gene therapy strategies and clinical applications. Discov Med. 2014;18:151–161. [PMC free article] [PubMed] [Google Scholar]
- 253.Sander JD, Joung JK. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat Biotechnol. 2014;32:347–355. doi: 10.1038/nbt.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Bao X, Zeng J, Huang H, Ma C, Wang L, Wang F, Liao X, Song X. Cancer-targeted PEDF-DNA therapy for metastatic colorectal cancer. Int J Pharm. 2020;576:118999. doi: 10.1016/j.ijpharm.2019.118999. [DOI] [PubMed] [Google Scholar]
- 255.Duffy AG, Makarova-Rusher OV, Ulahannan SV, Rahma OE, Fioravanti S, Walker M, Abdullah S, Raffeld M, Anderson V, Abi-Jaoudeh N, Levy E, Wood BJ, Lee S, Tomita Y, Trepel JB, Steinberg SM, Revenko AS, MacLeod AR, Peer CJ, Figg WD, Greten TF. Modulation of tumor eIF4E by antisense inhibition: A phase I/II translational clinical trial of ISIS 183750-an antisense oligonucleotide against eIF4E-in combination with irinotecan in solid tumors and irinotecan-refractory colorectal cancer. Int J Cancer. 2016;139:1648–1657. doi: 10.1002/ijc.30199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Mohammadi A, Mansoori B, Baradaran B. The role of microRNAs in colorectal cancer. Biomed Pharmacother. 2016;84:705–713. doi: 10.1016/j.biopha.2016.09.099. [DOI] [PubMed] [Google Scholar]
- 257.Zheng B, Mai Q, Jiang J, Zhou Q. The Therapeutic Potential of Small Activating RNAs for Colorectal Carcinoma. Curr Gene Ther. 2019;19:140–146. doi: 10.2174/1566523219666190708111404. [DOI] [PubMed] [Google Scholar]
- 258.Titze-de-Almeida R, David C, Titze-de-Almeida SS. The Race of 10 Synthetic RNAi-Based Drugs to the Pharmaceutical Market. Pharm Res. 2017;34:1339–1363. doi: 10.1007/s11095-017-2134-2. [DOI] [PubMed] [Google Scholar]
- 259.Yuan TL, Fellmann C, Lee CS, Ritchie CD, Thapar V, Lee LC, Hsu DJ, Grace D, Carver JO, Zuber J, Luo J, McCormick F, Lowe SW. Development of siRNA payloads to target KRAS-mutant cancer. Cancer Discov. 2014;4:1182–1197. doi: 10.1158/2159-8290.CD-13-0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Kleinstiver BP, Pattanayak V, Prew MS, Tsai SQ, Nguyen NT, Zheng Z, Joung JK. High-fidelity CRISPR-Cas9 nucleases with no detectable genome-wide off-target effects. Nature. 2016;529:490–495. doi: 10.1038/nature16526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Seki A, Rutz S. Optimized RNP transfection for highly efficient CRISPR/Cas9-mediated gene knockout in primary T cells. J Exp Med. 2018;215:985–997. doi: 10.1084/jem.20171626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Zhao Y, Xu J, Le VM, Gong Q, Li S, Gao F, Ni L, Liu J, Liang X. EpCAM Aptamer-Functionalized Cationic Liposome-Based Nanoparticles Loaded with miR-139-5p for Targeted Therapy in Colorectal Cancer. Mol Pharm. 2019;16:4696–4710. doi: 10.1021/acs.molpharmaceut.9b00867. [DOI] [PubMed] [Google Scholar]
- 263.Aghamiri S, Jafarpour A, Malekshahi ZV, Mahmoudi Gomari M, Negahdari B. Targeting siRNA in colorectal cancer therapy: Nanotechnology comes into view. J Cell Physiol. 2019;234:14818–14827. doi: 10.1002/jcp.28281. [DOI] [PubMed] [Google Scholar]
- 264.Sousa AR, Oliveira AV, Oliveira MJ, Sarmento B. Nanotechnology-based siRNA delivery strategies for metastatic colorectal cancer therapy. Int J Pharm. 2019;568:118530. doi: 10.1016/j.ijpharm.2019.118530. [DOI] [PubMed] [Google Scholar]
- 265.Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov. 2009;8:129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Zhou J, Patel TR, Fu M, Bertram JP, Saltzman WM. Octa-functional PLGA nanoparticles for targeted and efficient siRNA delivery to tumors. Biomaterials. 2012;33:583–591. doi: 10.1016/j.biomaterials.2011.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Li X, Bhullar AS, Binzel DW, Guo P. The dynamic, motile and deformative properties of RNA nanoparticles facilitate the third milestone of drug development. Adv Drug Deliv Rev. 2022;186:114316. doi: 10.1016/j.addr.2022.114316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Rychahou P, Haque F, Shu Y, Zaytseva Y, Weiss HL, Lee EY, Mustain W, Valentino J, Guo P, Evers BM. Delivery of RNA nanoparticles into colorectal cancer metastases following systemic administration. ACS Nano. 2015;9:1108–1116. doi: 10.1021/acsnano.5b00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Binzel DW, Guo S, Yin H, Lee TJ, Liu S, Shu D, Guo P. Rational design for controlled release of Dicer-substrate siRNA harbored in phi29 pRNA-based nanoparticles. Mol Ther Nucleic Acids. 2021;25:524–535. doi: 10.1016/j.omtn.2021.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Wan T, Pan Q, Liu C, Guo J, Li B, Yan X, Cheng Y, Ping Y. A Duplex CRISPR-Cas9 Ribonucleoprotein Nanomedicine for Colorectal Cancer Gene Therapy. Nano Lett. 2021;21:9761–9771. doi: 10.1021/acs.nanolett.1c03708. [DOI] [PubMed] [Google Scholar]
- 271.Zhiani M, Mousavi MA, Rostamizadeh K, Pirizadeh R, Osali A, Mennati A, Motlagh B, Fathi M. Apoptosis induction by siRNA targeting integrin-β1 and regorafenib/DDAB-mPEG-PCL hybrid nanoparticles in regorafenib-resistant colon cancer cells. Am J Cancer Res. 2021;11:1170–1184. [PMC free article] [PubMed] [Google Scholar]
- 272.Palko-Labuz A, Sroda-Pomianek K, Uryga A, Kostrzewa-Suslow E, Michalak K. Anticancer activity of baicalein and luteolin studied in colorectal adenocarcinoma LoVo cells and in drug-resistant LoVo/Dx cells. Biomed Pharmacother. 2017;88:232–241. doi: 10.1016/j.biopha.2017.01.053. [DOI] [PubMed] [Google Scholar]
- 273.Fang JK, Chen L, Lu XG, Cao D, Guo LL, Zhang YS, Li LB, Zhang LF, Kuang YT, Wang SL. Optimization of Transforming Growth Factor-β1 siRNA Loaded Chitosan-Tripolyphosphate Nanoparticles for the Treatment of Colorectal Cancer Hepatic Metastasis in a Mouse Model. J Biomed Nanotechnol. 2016;12:1489–1500. doi: 10.1166/jbn.2016.2265. [DOI] [PubMed] [Google Scholar]
- 274.Li L, Gu W, Chen J, Chen W, Xu ZP. Co-delivery of siRNAs and anti-cancer drugs using layered double hydroxide nanoparticles. Biomaterials. 2014;35:3331–3339. doi: 10.1016/j.biomaterials.2013.12.095. [DOI] [PubMed] [Google Scholar]
- 275.Li T, Wan Y, Su Z, Li J, Han M, Zhou C. Mesenchymal Stem Cell-Derived Exosomal microRNA-3940-5p Inhibits Colorectal Cancer Metastasis by Targeting Integrin α6. Dig Dis Sci. 2021;66:1916–1927. doi: 10.1007/s10620-020-06458-1. [DOI] [PubMed] [Google Scholar]
- 276.Liu L, Yu T, Jin Y, Mai W, Zhou J, Zhao C. MicroRNA-15a Carried by Mesenchymal Stem Cell-Derived Extracellular Vesicles Inhibits the Immune Evasion of Colorectal Cancer Cells by Regulating the KDM4B/HOXC4/PD-L1 Axis. Front Cell Dev Biol. 2021;9:629893. doi: 10.3389/fcell.2021.629893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Xie YH, Chen YX, Fang JY. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct Target Ther. 2020;5:22. doi: 10.1038/s41392-020-0116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Lieu CH, Corcoran RB, Overman MJ. Integrating Biomarkers and Targeted Therapy Into Colorectal Cancer Management. Am Soc Clin Oncol Educ Book. 2019;39:207–215. doi: 10.1200/EDBK_240839. [DOI] [PubMed] [Google Scholar]
- 279.Matos AI, Carreira B, Peres C, Moura LIF, Conniot J, Fourniols T, Scomparin A, Martínez-Barriocanal Á, Arango D, Conde JP, Préat V, Satchi-Fainaro R, Florindo HF. Nanotechnology is an important strategy for combinational innovative chemo-immunotherapies against colorectal cancer. J Control Release. 2019;307:108–138. doi: 10.1016/j.jconrel.2019.06.017. [DOI] [PubMed] [Google Scholar]
- 280.Silveira MJ, Castro F, Oliveira MJ, Sarmento B. Immunomodulatory nanomedicine for colorectal cancer treatment: a landscape to be explored? Biomater Sci. 2021;9:3228–3243. doi: 10.1039/d1bm00137j. [DOI] [PubMed] [Google Scholar]
- 281.Ni Q, Zhang F, Liu Y, Wang Z, Yu G, Liang B, Niu G, Su T, Zhu G, Lu G, Zhang L, Chen X. A bi-adjuvant nanovaccine that potentiates immunogenicity of neoantigen for combination immunotherapy of colorectal cancer. Sci Adv. 2020;6:eaaw6071. doi: 10.1126/sciadv.aaw6071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Luo L, Yang J, Zhu C, Jiang M, Guo X, Li W, Yin X, Yin H, Qin B, Yuan X, Li Q, Du Y, You J. Sustained release of anti-PD-1 peptide for perdurable immunotherapy together with photothermal ablation against primary and distant tumors. J Control Release. 2018;278:87–99. doi: 10.1016/j.jconrel.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 283.He C, Duan X, Guo N, Chan C, Poon C, Weichselbaum RR, Lin W. Core-shell nanoscale coordination polymers combine chemotherapy and photodynamic therapy to potentiate checkpoint blockade cancer immunotherapy. Nat Commun. 2016;7:12499. doi: 10.1038/ncomms12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Lu K, He C, Guo N, Chan C, Ni K, Lan G, Tang H, Pelizzari C, Fu YX, Spiotto MT, Weichselbaum RR, Lin W. Low-dose X-ray radiotherapy-radiodynamic therapy via nanoscale metal-organic frameworks enhances checkpoint blockade immunotherapy. Nat Biomed Eng. 2018;2:600–610. doi: 10.1038/s41551-018-0203-4. [DOI] [PubMed] [Google Scholar]
- 285.Lan H, Zhang W, Jin K, Liu Y, Wang Z. Modulating barriers of tumor microenvironment through nanocarrier systems for improved cancer immunotherapy: a review of current status and future perspective. Drug Deliv. 2020;27:1248–1262. doi: 10.1080/10717544.2020.1809559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Martin JD, Cabral H, Stylianopoulos T, Jain RK. Improving cancer immunotherapy using nanomedicines: progress, opportunities and challenges. Nat Rev Clin Oncol. 2020;17:251–266. doi: 10.1038/s41571-019-0308-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Yin WM, Li YW, Gu YQ, Luo M. Nanoengineered targeting strategy for cancer immunotherapy. Acta Pharmacol Sin. 2020;41:902–910. doi: 10.1038/s41401-020-0417-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Zhu G, Mei L, Vishwasrao HD, Jacobson O, Wang Z, Liu Y, Yung BC, Fu X, Jin A, Niu G, Wang Q, Zhang F, Shroff H, Chen X. Intertwining DNA-RNA nanocapsules loaded with tumor neoantigens as synergistic nanovaccines for cancer immunotherapy. Nat Commun. 2017;8:1482. doi: 10.1038/s41467-017-01386-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Song W, Tiruthani K, Wang Y, Shen L, Hu M, Dorosheva O, Qiu K, Kinghorn KA, Liu R, Huang L. Trapping of Lipopolysaccharide to Promote Immunotherapy against Colorectal Cancer and Attenuate Liver Metastasis. Adv Mater. 2018;30:e1805007. doi: 10.1002/adma.201805007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Javadi S, Rostamizadeh K, Hejazi J, Parsa M, Fathi M. Curcumin mediated down-regulation of α(V) β(3) integrin and up-regulation of pyruvate dehydrogenase kinase 4 (PDK4) in Erlotinib resistant SW480 colon cancer cells. Phytother Res. 2018;32:355–364. doi: 10.1002/ptr.5984. [DOI] [PubMed] [Google Scholar]
- 291.El Hallal R, Lyu N, Wang Y. Effect of Cetuximab-Conjugated Gold Nanoparticles on the Cytotoxicity and Phenotypic Evolution of Colorectal Cancer Cells. Molecules. 2021;26 doi: 10.3390/molecules26030567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Naseri M, Zöller M, Hadjati J, Ghods R, Ranaei Pirmardan E, Kiani J, Eini L, Bozorgmehr M, Madjd Z. Dendritic cells loaded with exosomes derived from cancer stem cell-enriched spheroids as a potential immunotherapeutic option. J Cell Mol Med. 2021;25:3312–3326. doi: 10.1111/jcmm.16401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Hosseini M, Baghaei K, Hajivalili M, Zali MR, Ebtekar M, Amani D. The anti-tumor effects of CT-26 derived exosomes enriched by MicroRNA-34a on murine model of colorectal cancer. Life Sci. 2022;290:120234. doi: 10.1016/j.lfs.2021.120234. [DOI] [PubMed] [Google Scholar]
- 294.Kobayashi H, Enomoto A, Woods SL, Burt AD, Takahashi M, Worthley DL. Cancer-associated fibroblasts in gastrointestinal cancer. Nat Rev Gastroenterol Hepatol. 2019;16:282–295. doi: 10.1038/s41575-019-0115-0. [DOI] [PubMed] [Google Scholar]
- 295.Loeffler M, Krüger JA, Niethammer AG, Reisfeld RA. Targeting tumor-associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake. J Clin Invest. 2006;116:1955–1962. doi: 10.1172/JCI26532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Ferrer-Mayorga G, Gómez-López G, Barbáchano A, Fernández-Barral A, Peña C, Pisano DG, Cantero R, Rojo F, Muñoz A, Larriba MJ. Vitamin D receptor expression and associated gene signature in tumour stromal fibroblasts predict clinical outcome in colorectal cancer. Gut. 2017;66:1449–1462. doi: 10.1136/gutjnl-2015-310977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Ji T, Zhao Y, Ding Y, Wang J, Zhao R, Lang J, Qin H, Liu X, Shi J, Tao N, Qin Z, Nie G. Transformable Peptide Nanocarriers for Expeditious Drug Release and Effective Cancer Therapy via Cancer-Associated Fibroblast Activation. Angew Chem Int Ed Engl. 2016;55:1050–1055. doi: 10.1002/anie.201506262. [DOI] [PMC free article] [PubMed] [Google Scholar]