Abstract
Background
Few studies have investigated associations of acclerometer‐based assessments of physical activity (PA) and sedentary behavior (SB) with incidence of cardiovascular disease (CVD) and its components. This prospective cohort study assessed the associations of accelerometer‐measured PA and SB with total CVD, myocardial infarction, and ischemic stroke (IS).
Methods and Results
The authors included 16 031 women aged 62 years and older, free of CVD, with adherent accelerometer wear (≥10 hours/day for ≥4 days) from the Women's Health Study (mean age, 71.4 years [SD, 5.6 years]). Hip‐worn ActiGraph GT3X+ accelerometers measured total volume of PA (total average daily vector magnitude), minutes per day of high‐light PA and moderate to vigorous PA (MVPA), and SB. Women reported diagnoses of CVD, which were adjudicated using medical records and death certificates. Hazard ratios (HRs) were estimated for each exposure, and 95% CIs using Cox proportional hazards models were adjusted for accelerometer wear time, age, self‐reported general health, postmenopausal hormone therapy, smoking status, and alcohol use. The hypothetical effect of replacing 10 minutes/day of SB or high‐light PA with MVPA on CVD incidence was assessed using adjusted isotemporal substitution Cox models. Over a mean of 7.1 years (SD, 1.6 years) of follow‐up, 482 total CVD cases, 107 myocardial infarction cases, and 181 IS cases were diagnosed. Compared with the lowest quartiles of total average daily vector magnitude and MVPA (≤60 minutes), women who were in the highest quartiles (>120 minutes of MVPA) had a 43% (95% CI, 24%–58%) and 38% (95% CI, 18%–54%) lower hazard of total CVD, respectively. Estimates were similar for total average daily vector magnitude and MVPA with IS, but PA was not associated with myocardial infarction overall. High‐light PA was not associated with any CVD outcomes. Women who spent <7.4 hours sedentary per day had a 33% (95% CI, 11%–49%) lower hazard of total CVD compared with those who spent ≥9.5 hours sedentary. Replacing 10 minutes of SB with MVPA was associated with a 4% lower incidence of total CVD (HR, 0.96 [95% CI, 0.93–0.99]).
Conclusions
Accelerometer‐assessed total PA and MVPA were inversely associated with total CVD and IS incidence, and SB was directly associated with total CVD; high‐light PA was not related to CVD.
Keywords: accelerometry, cardiovascular disease, epidemiology, ischemic stroke, myocardial infarction, physical activity, sedentary behavior
Subject Categories: Cardiovascular Disease, Epidemiology, Exercise, Lifestyle, Risk Factors
Nonstandard Abbreviations and Acronyms
- HLPA
high‐light physical activity
- IS
ischemic stroke
- LLPA
low‐light physical activity
- MVPA
moderate to vigorous physical activity
- PA
physical activity
- SB
sedentary behavior
- VM
vector magnitude
- WHS
Women's Health Study
Clinical Perspective.
What Is New?
Few data are available on the associations of device‐assessed physical activity (PA) and sedentary behavior (SB) with total cardiovascular disease (CVD), myocardial infarction, and ischemic stroke. Devices can measure light‐intensity PA, which makes up a large proportion of PA among older adults.
In this sample of older women, greater total PA and moderate to vigorous PA were associated with lower incidence of total CVD and ischemic stroke but not myocardial infarction; however, high‐light PA was not associated with any CVD outcomes.
Less time spent in SB was associated with lower total CVD incidence but not myocardial infarction or ischemic stroke.
What Are the Clinical Implications?
Current guidelines emphasize the importance of moderate to vigorous PA and less SB in reducing the risk of CVD; this study supports these guideline recommendations.
Greater time spent in SB was associated with higher total CVD incidence.
Replacing 10 minutes/day of SB with 10 minutes of moderate to vigorous PA was associated with a 4% lower total CVD incidence.
In 2021, cardiovascular disease (CVD) was the leading cause of death in the world, amounting to 32% of all mortality. 1 Compared with 2010, the number of deaths attributable to CVD worldwide in 2020 increased by almost 19%, amounting to ≈19 million deaths. 2 CVD increases with age, particularly among older women, 3 but it is estimated that ≈80% of CVD is preventable through management of risk factors such as smoking, poor diet, high blood pressure, elevated lipid levels, and low levels of physical activity (PA). 3 Moderate to vigorous PA (MVPA) assessed by self‐report has been consistently associated with a lower risk of incident CVD and CVD mortality. 4 Conversely, greater time spent in self‐reported sedentary behavior (SB) may be associated with an elevated risk of incident CVD, independent of PA. 4 , 5
Current US recommendations for adults are primarily based on findings from self‐reported PA data with a focus on absolute‐intensity MVPA (≥3.0 metabolic equivalents). 6 Typically, MVPA decreases with age, 7 as some individuals may not be able to participate in higher‐intensity PAs. 8 Cumulative “high‐light” PA (HLPA), a subset of light PA consisting of purposeful movements such as slow walking, shopping, or household work (2.3–2.9 metabolic equivalents), 6 should be investigated as an attainable alternative to MVPA for preventing CVD in older populations. 9 Additionally, accelerometry data indicate that adults aged 60 to 75 years spend more time in SB than their younger counterparts, with SB accounting for ≈12.3 hours of a 16.0‐hour awake day. 10 Self‐reported measures do not capture light‐intensity PA well, 11 tend to underestimate time spent in SB, 12 and are impacted by both recall bias and social desirability bias. 13 Accelerometers present the opportunity to further research the associations of daily activities, particularly light PA and SB, with risk of CVD in older populations.
More recent investigations using accelerometry have mostly confirmed the established relationship of MVPA with incident CVD 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 and CVD mortality 19 , 22 , 23 , 24 , 25 , 26 previously found using self‐reported data, but with larger magnitudes of association. 11 However, accelerometry studies provide mixed evidence on the importance of light PA for CVD risk. 14 , 15 , 16 , 17 , 18 , 19 , 23 , 24 , 25 , 26 Moreover, although there is strong evidence for an association between self‐reported SB and CVD incidence, 11 there is mixed evidence for whether accelerometer‐measured SB is associated with an increased risk of CVD or CVD mortality. 14 , 15 , 16 , 18 , 22 , 24 , 25 , 27 , 28 Public health interventions that increase time spent in higher‐intensity activities inherently require spending less time in a lower‐intensity activity, as time is fixed within a day. Few studies have assessed whether substituting time spent sedentary for either HLPA or MVPA, measured by accelerometry, is associated with lower risk of CVD. 21 , 24 , 25 In addition, little is known about accelerometry‐derived measures and their relationships to CVD components such as myocardial infarction (MI) and ischemic stroke (IS). To our knowledge, no prior studies have investigated accelerometry with MI incidence, and only 3 with stroke incidence. 19 , 20 , 29
We investigated the association of accelerometer‐measured total average daily volume of PA, MVPA, HLPA, and SB with time to incident total CVD, MI, and IS in WHS (Women's Health Study). Additionally, we explored the association of substituting time spent in lower intensities of activity (SB or HLPA) for time in higher‐intensity activities (HLPA or MVPA), with time to incidence of total CVD, MI, and IS.
METHODS
Study Design and Population
The data that support the findings of this study are available from the corresponding author upon reasonable request. WHS was a randomized clinical trial that ran from 1992 to 2004, examining the effects of vitamin E and low‐dose aspirin for the prevention of cancer and CVD among 39 876 women 45 years and older living in the United States. 30 , 31 , 32 At the end of the trial, participants were invited to join an observational study, and 33 682 (89% of survivors) consented. Between 2011 and 2015, WHS survivors were invited to participate in an ancillary accelerometry study, with 17 707 wearing and returning their devices; details have been previously published. 33 For the present analysis, 16 031 participants 62 years and older and free of CVD (n=712 with prevalent CVD), who were compliant with the device wear protocol (see below for details) 33 , 34 , 35 , 36 , 37 were included. All enrolled women provided written informed consent and this study received approval from the institutional review board of the Brigham and Women's Hospital.
Accelerometer‐Measured PA and SB
Women were mailed a triaxial accelerometer (ActiGraph GT3X+) to be worn on the right hip during waking hours for 7 days. Accelerometer details have been previously outlined. 33 Briefly, accelerometer nonwear was defined using the validated Choi et al algorithm. 36 , 38 Vector magnitude (VM), the measure of movement (acceleration) derived from triaxial accelerometers, is defined as the square root of the sum of the squares from 3 axis planes of movement: Total daily volume of PA (total average daily VM) and daily minutes spent in MVPA, HLPA, and SB were used in this analysis. PA intensities and SB were defined using previously identified cut points calibrated in older adults: time in SB as <19 VM per 15‐second epoch, “low‐light” PA (LLPA) as 19 to 225 VM per 15‐second epoch, HLPA as 226 to 518 VM per 15‐second epoch, and MVPA as ≥519 VM per 15‐second epoch. 6 We chose to investigate HLPA as an exposure, as opposed to total light PA or LLPA, as there is some degree of measurement error and misclassification between SB and LLPA. 6 Furthermore, when differentiated between HLPA and LLPA, only HLPA was found to be associated with CVD mortality in a study of similarly aged women. 23
CVD Outcomes
Time to occurrence of total CVD, MI, and IS were examined as separate outcomes. Total CVD was defined as the first occurrence of MI, IS, hemorrhagic stroke, coronary artery bypass grafting, percutaneous transluminal coronary angioplasty, or CVD death without antecedent history of CVD. CVD events were self‐reported on annual questionnaires and confirmed by medical record and death certificate review; total CVD included CVD deaths and events that were never self‐reported as well. Only confirmed cases were included in the analyses. Established criteria were used to confirm incidence of MI and stroke. 39 , 40 If sufficient evidence for a death caused by a cardiovascular event was provided by all available sources, CVD death was determined. 41 Although MI and IS share many cardiovascular risk factors and are both commonly caused by atherosclerotic disease, few patients experience both MI and IS (≈<4% of patients with MI experience a subsequent stroke) 42 ; therefore, we investigated time to MI and IS as separate outcomes as only 2 participants in our population experienced both an MI and an IS during follow‐up. This analysis investigated IS only as an outcome, rather than a combined total of IS and hemorrhagic stroke, as the biological mechanisms connecting PA and stroke may differ for these subtypes. 43 Additionally, the number of hemorrhagic strokes was deemed too small for meaningful analyses as a separate end point. Participants who did not experience a CVD event during the follow‐up period were considered censored at the last date of follow‐up.
Covariates
Participants reported sociodemographic and health information on yearly, mailed questionnaires. Questionnaires administered closest to accelerometer collection were used in this analysis, except for education (≥4‐year degree, <4‐year degree), age (years), energy‐adjusted saturated fat intake (g/d), energy‐adjusted fiber intake (g/d), and fruit and vegetable composite score (servings per day), which were assessed on the baseline questionnaire for the clinical trial. Self‐reported smoking status (current, former, or never), alcohol use (rarely, monthly, weekly, or daily), general health status (excellent, very good, good, or fair/poor), postmenopausal hormone therapy use (never, past, or current), history of cancer (yes or no), history of hypertension (yes or no), history of high cholesterol (yes or no), history of diabetes (yes or no), parental history of MI (yes or no), body mass index (BMI; calculated as weight in kilograms divided by height in meters sqared), and physical function measured using a 10‐item subscale of the RAND 36‐Item Health Survey 1.0 (with a range of 0 [low] to 100 [high] 44 ) were taken from the yearly follow‐up questionnaire closest to and following the accelerometer collection.
Statistical Analysis
We compared sociodemographic and health‐related characteristics of women across quartiles of total daily volume of PA. Through the use of directed acyclic graphs, we identified accelerometer wear time, age, self‐reported general health, postmenopausal hormone therapy use, smoking status, and alcohol use as potential confounders (Figure S1). We assessed whether PA and SB associations were nonlinear by comparing restricted cubic splines with conventional knots at the 5th, 50th, and 95th percentiles 45 , 46 with a linear specification by using the likelihood ratio test (α<0.05). For all PA and SB variables, spline terms were not significant. Therefore, we executed 2 analyses: one that used categorical quartiles of PA and SB variables, and another that used continuous, linear specifications of the exposure variables. In addition, we modeled PA and SB as ordinal quartiles to conduct P for trend tests; however, hazard ratios (HRs) for quartiles were estimated from nominal quartiles.
We calculated age‐adjusted rates of CVD per 100 000 person‐years using Poisson regressions by quartiles of PA and SB. We used Cox proportional hazards models to estimate associations of accelerometer‐measured PA and SB with time to total CVD, MI, and IS. We assessed the proportional hazards assumption by including an interaction between independent variables and the log of time; none of the exposure measures violated this assumption. Model 1 estimated HRs of incident CVD for PA intensity and SB, adjusting for potential confounders. We included BMI, history of hypertension, history of high cholesterol, diabetes, and physical function as potential mediators that may also be confounders (see DAG in Figure S1) in model 2, in addition to confounders identified for model 1. A quadratic term was included for the representation of physical function in model 2.
We identified self‐reported general health status, age, obesity, and physical function 17 a priori as potential effect measure modifiers. We also investigated whether the associations between MVPA and time to CVD outcomes were modified by SB, and whether these associations with SB were modified by MVPA. We assessed effect measure modification by exploring the distributions of exposures and outcomes by the modifier, investigating stratum‐specific estimates by the modifier using model 1 covariates, and by the likelihood ratio test for inclusion of modifier interaction terms in model 1. Modification was primarily determined by P values for interaction terms produced by the likelihood ratio test but was also based on the degree to which stratum‐specific estimates differed and their CI overlap.
We were interested in the hypothetical effect of replacing time spent in one behavior (SB or HLPA) with time in another (HLPA or MVPA) and its association with time to CVD, as public health interventions that encourage an increase in MVPA inherently reduce time spent in another activity due to the fixed nature of time. We investigated the associations of replacing either 10 or 30 minutes of SB or HLPA with 10 or 30 minutes of HLPA or MVPA and time to CVD, using isotemporal substitution Cox models adjusted for model 1 confounders. 47 Any minute bout counted; PA was not required to be in 10‐minute bouts. The choice of 10 or 30‐minute substitutions was made for ease of interpretation of models.
To assess the role of bias from confounding attributable to prior health status, sensitivity analyses excluded all events within the first year of follow‐up and women who self‐reported fair/poor general health at the time of accelerometry collection, as PA and SB may be reflective of a declining state of health rather than predictive of future disease risk for these women. 48 Data were analyzed using SAS 9.4 (SAS Institute Inc), and graphics were made in R (version 3.5.1; R Foundation for Statistical Computing).
RESULTS
Participant Characteristics
Participants had a mean age of 71.4 years (SD, 5.6 years), mean BMI of 26.2 (SD, 5.0), and 46% were former and 51% never smokers (Table 1). Of the participants, 67% had a history of hypertension, 72% had a history of high cholesterol, and 8% had a history of diabetes. Median accelerometer wear time was 14.9 hours (interquartile range [IQR], 14.1–15.6 hours) per day, with women engaging in a median of 88 minutes (IQR, 60–120 minutes) of MVPA per day, 107 minutes (IQR, 86–129 minutes) of HLPA per day, and 508 minutes (IQR, 442–574 minutes) of SB per day (Table 2).
Table 1.
Sociodemographic and Health‐Related Characteristics of the Study Population by Quartiles of Daily Total Volume of Activity (Accelerometer‐Measured Total VM) (N=16 031)
| Overall | Quartiles of total VM | ||||
|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||
| Mean (SD) age, y | 71.4 (5.6) | 73.8 (6.3) | 71.8 (5.5) | 70.6 (5.1) | 69.4 (4.4) |
| Mean (SD) BMI, kg/m2 | 26.2 (5.0) | 28.4 (5.9) | 26.6 (4.8) | 25.4 (4.3) | 24.2 (3.7) |
| Smoking status, n (%) | |||||
| Current | 559 (3) | 202 (5) | 145 (4) | 116 (3) | 95 (2) |
| Former | 7352 (46) | 1848 (46) | 1855 (46) | 1873 (47) | 1776 (44) |
| Never | 8119 (51) | 1957 (49) | 2007 (50) | 2018 (50) | 2137 (53) |
| Alcohol use, n (%) | |||||
| Rarely | 6023 (38) | 1810 (45) | 1514 (38) | 1401 (35) | 1298 (32) |
| Monthly | 1584 (10) | 428 (11) | 403 (10) | 381 (10) | 372 (9) |
| Weekly | 5846 (36) | 1241 (31) | 1494 (37) | 1495 (37) | 1616 (40) |
| Daily | 2572 (16) | 526 (13) | 597 (15) | 727 (18) | 722 (18) |
| Education, n (%) | |||||
| <4‐y degree | 7776 (49) | 2155 (55) | 1929 (49) | 1838 (47) | 1854 (47) |
| ≥4‐y degree | 7996 (51) | 1795 (45) | 2010 (51) | 2097 (53) | 2094 (53) |
| History of hypertension, n (%) | 10 758 (67) | 3267 (82) | 2801 (70) | 2523 (63) | 2167 (54) |
| History of high cholesterol, n (%) | 11 606 (72) | 3160 (79) | 2983 (74) | 2844 (71) | 2619 (66) |
| History of diabetes, n (%) | 1337 (8) | 622 (16) | 338 (8) | 229 (6) | 148 (4) |
| Self‐reported general health, n (%) | |||||
| Excellent | 4057 (25) | 564 (14) | 915 (23) | 1166 (29) | 1412 (35) |
| Very good | 8096 (51) | 1930 (48) | 2074 (52) | 2093 (52) | 1999 (50) |
| Good | 3520 (22) | 1318 (33) | 943 (24) | 698 (17) | 561 (14) |
| Fair/poor | 353 (2) | 194 (5) | 75 (2) | 48 (1) | 36 (1) |
| Hormone therapy use, n (%) | |||||
| Never | 2901 (18) | 841 (21) | 697 (17) | 684 (17) | 674 (17) |
| Past | 11 507 (72) | 2819 (70) | 2904 (72) | 2871 (72) | 2913 (73) |
| Current | 1617 (10) | 345 (9) | 407 (10) | 450 (11) | 415 (10) |
| History of cancer, n (%) | 1906 (12) | 547 (14) | 520 (13) | 449 (11) | 390 (10) |
| Parental history of MI, n (%) | 2259 (14) | 595 (15) | 576 (15) | 529 (13) | 559 (14) |
| Mean (SD) physical function score | 80.9 (20.7) | 69.8 (24.2) | 80.2 (19.5) | 84.7 (18.0) | 88.3 (15.7) |
| Mean (SD) saturated fat intake, g/d | 20.5 (6.0) | 21.1 (6.2) | 20.4 (5.9) | 20.4 (6.0) | 19.9 (5.9) |
| Mean (SD) fiber intake, g/d | 22.3 (7.1) | 21.7 (6.9) | 22.2 (6.9) | 22.4 (7.6) | 22.9 (7.0) |
| Mean (SD) daily servings of fruits and vegetables | 6.7 (3.7) | 6.5 (3.7) | 6.7 (3.9) | 6.8 (3.7) | 6.9 (3.6) |
Quartiles of total vector magnitude (VM; 1000 s): quartile 1 ≤385.4; 385.4< quartile 2 ≤502.9; 502.9< quartile 3 ≤639.5; 639.5> quartile 4. BMI indicates body mass index; and MI, myocardial infarction.
Table 2.
Median (IQR) of Daily Accelerometer‐Measured PA and SB of the Study Population, by Quartiles of Daily Total Volume of Activity (Total VM) (N=16 031)
| Overall | Quartiles of total VM | ||||
|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||
| Accelerometer wear time, h/d | 14.9 (14.1–15.6) | 14.4 (13.6–15.2) | 14.8 (14.0–15.5) | 15.0 (14.3–15.7) | 15.3 (14.6–16.0) |
| Total VM, 1000 s | 502.9 (385.4–639.5) | 311.1 (259.6–351.4) | 444.8 (416.3–473.6) | 563.8 (533.1–599.4) | 751.2 (687.8–848.6) |
| MVPA, min/d | 87.5 (60.0–119.6) | 43.9 (32.1–55.1) | 74.5 (65.2–83.0) | 102.0 (92.4–111.7) | 145.5 (130.1–169.0) |
| HLPA, min/d | 106.6 (86.2–128.9) | 78.7 (65.0–93.1) | 102.4 (88.2–118.0) | 115.7 (99.4–133.9) | 132.2 (114.0–152.4) |
| SB, min/d | 507.9 (442.9–573.5) | 587.4 (535.2–639.7) | 529.8 (481.3–578.2) | 488.5 (442.1–537.5) | 426.1 (375.1–478.7) |
Quartiles of total vector magnitude (VM; 1000 s): quartile 1 ≤385.4; 385.4< quartile 2≤ 502.9; 502.9< quartile 3 ≤639.5; 639.5> quartile 4. HLPA indicates high‐light physical activity; IQR, interquartile range; MVPA, moderate to vigorous physical activity; SB, sedentary behavior.
Compared with women in the highest quartile of total volume of PA, women who completed the least amount of PA were on average 4 years older, included more smokers, self‐reported excellent general health less frequently, and had a greater BMI (Table 1). In addition, prevalence of chronic conditions such as cancer, hypertension, high cholesterol, and diabetes were higher among women in the lowest quartile of total volume of PA compared with the highest quartile.
During a mean of 7.2 years (SD, 1.6 years) of follow‐up, 482 total CVD events were confirmed; 107 of these events were MI and 181 were IS. Age‐adjusted rates of total CVD per 100 000 person‐years were greatest for lower levels of total average daily VM, MVPA, and HLPA, and were lower with successively higher groups of total volume of PA and MVPA (Table S1). This trend was less pronounced for HLPA than for total average daily VM and MVPA. Age‐adjusted rates of total CVD were lowest for lower levels of SB, and higher with greater minutes per day of SB.
Associations With Total CVD
Accumulating more total average daily VM and minutes of MVPA per day were associated with lower hazards of total CVD (Figure 1). Women who accumulated the highest quartile of VM had a 43% (HR, 0.57 [95% CI, 0.42–0.76]) lower hazard of total CVD compared with women in the lowest quartile of total average daily VM (Table S2). Women who spent ≥120 minutes per day in MVPA had a 38% (HR, 0.62 [95% CI, 0.46–0.82]) lower hazard of total CVD compared with women who spent <60 minutes per day in MVPA. HLPA was not associated with total CVD. Compared with accumulating ≥9.5 hours of SB per day, accumulating <7.4 hours of SB per day was associated with a 33% (HR, 0.67 [95% CI, 0.51–0.89]) lower hazard of total CVD. Similar trends were observed with continuous measures of total average daily VM, MVPA, HLPA, and SB.
Figure 1. Hazard ratios of total cardiovascular disease (CVD) events for increasing quartiles of physical activity (PA) and sedentary behavior, compared with the lowest quartile of PA and the highest quartile of sedentary behavior.
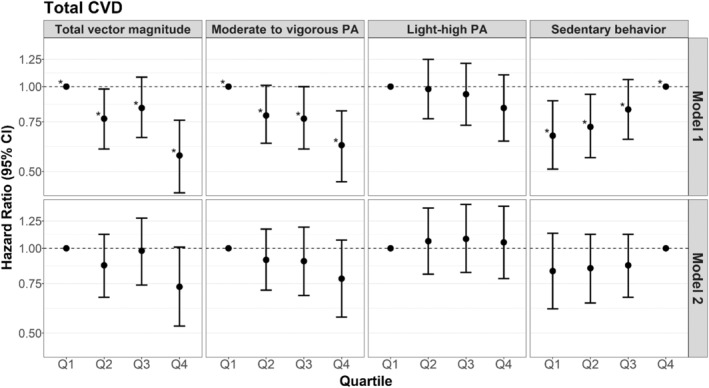
Q1 indicates quartile 1; Q2, quartile 2; Q3, quartile 3; and Q4, quartile 4. *P for trend <0.05 when modeled as an ordinal variable. Model 1: Cox proportional hazards models adjusted for confounders: accelerometer wear time, age, self‐reported general health, postmenopausal hormone therapy, smoking status, and alcohol use. Model 2: adjusted for confounders+potential mediators (body mass index, hypertension, high cholesterol, diabetes, and physical function). Corresponding numeric estimates, number of events for each quartile, and quartile threshold values can be found in Table S2.
Associations With MI
Compared with the lowest quartiles, the upper quartiles of total average daily VM (HR, 0.73 [95% CI, 0.40–1.33]) and minutes per day of MVPA (HR, 0.81 [95% CI, 0.44–1.47]) were associated with a nonsignificant lower hazard of MI, but CIs were wide (Figure 2, Table S2). We did not find evidence that HLPA was associated with hazards of MI. Accumulation of ≤7.4 hours of SB per day was associated with a nonsignificant lower hazard of MI (HR, 0.65 [95% CI, 0.36–1.17]) compared with ≥9.5 hours of SB per day; however, estimates were imprecise. Continuous variable analyses showed similar patterns with wide CIs.
Figure 2. Hazard ratios of myocardial infarction (MI) for increasing quartiles of physical activity (PA) and sedentary behavior, compared with the lowest quartile of PA and the highest quartile of sedentary behavior.
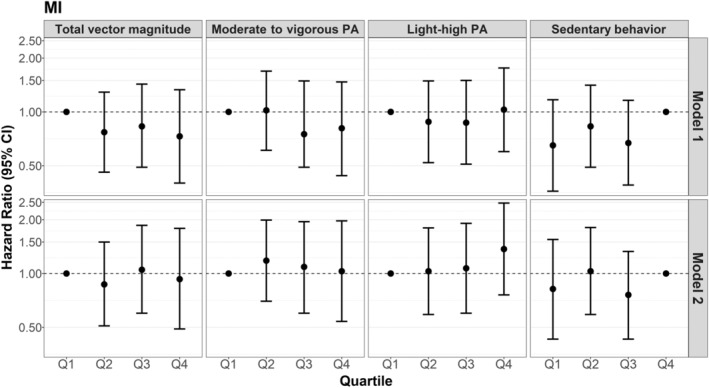
Q1 indicates quartile 1; Q2, quartile 2; Q3, quartile 3; and Q4, quartile 4. *P for trend <0.05 when modeled as an ordinal variable. Model 1: Cox proportional hazards models adjusted for confounders: accelerometer wear time, age, self‐reported general health, postmenopausal hormone therapy, smoking status, and alcohol use. Model 2: adjusted for confounders+potential mediators (body mass index, hypertension, high cholesterol, diabetes, and physical function). Corresponding numeric estimates, number of events for each quartile, and quartile threshold values can be found in Table S2.
Associations With IS
Greater total average daily VM and minutes per day of MVPA were associated with lower hazards of IS (Figure 3). Women who accumulated the highest quartile of total average daily VM had a 41% (HR, 0.59 [95% CI, 0.37–0.97]) lower hazard of IS compared with women in the lowest quartile of total average daily VM (Table S2). Participants who completed ≥120 minutes of MVPA per day also had a 41% (HR, 0.59 [95% CI, 0.36–0.94]) lower hazard of IS, compared with those who completed ≤60 minutes of MVPA. HLPA was not associated with IS. Compared with women who accumulated ≥9.5 hours of SB per day, those with <7.4 hours were at a nonstatistically significantly lower hazard of IS (HR, 0.85 [95% CI, 0.55–1.33]). Continuous variable analyses showed similar associations with wide CIs.
Figure 3. Hazard ratios of ischemic stroke for increasing quartiles of physical activity (PA) and sedentary behavior, compared with the lowest quartile of PA and the highest quartile of sedentary behavior.
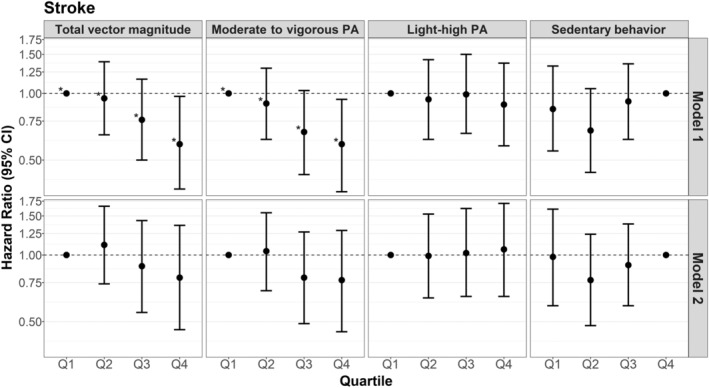
Q1 indicates quartile 1; Q2, quartile 2; Q3, quartile 3; and Q4, quartile 4. *P for trend <0.05 when modeled as an ordinal variable. Model 1: Cox proportional hazards models adjusted for confounders: accelerometer wear time, age, self‐reported general health, postmenopausal hormone therapy, smoking status, and alcohol use. Model 2: adjusted for confounders+potential mediators (body mass index, hypertension, high cholesterol, diabetes, and physical function). Corresponding numeric estimates, number of events for each quartile, and quartile threshold values can be found in Table S2.
Generally, findings from the addition of model 2 potential mediators to model 1 attenuated observed associations and yielded wider CIs, while typically maintaining the same direction of the association observed in model 1 for total CVD, MI, and IS (Figures 1, 2, 3, Table S2).
Isotemporal Substitution Models
Isotemporal models revealed that replacement of 10 minutes per day of SB with MVPA was associated with a lower hazard of total CVD (HR, 0.96 [95% CI, 0.93–0.99]) (Table 3). Similar estimates were found for MI (HR, 0.95 [95% CI, 0.89–1.01]) and IS (HR, 0.96 [95% CI, 0.92–1.00]) with wider CIs, which may reflect limited statistical power for the individual CVD components. The replacement of 10 minutes per day of SB with HLPA was not associated with lower hazards of total CVD (HR, 1.02 [95% CI, 0.98–1.06]), MI (HR, 1.04 [95% CI, 0.95–1.13]), or IS (HR, 1.03 [95% CI, 0.96–1.10]). Substituting HLPA with 10 minutes per day of MVPA was related to lower hazards of total CVD (HR, 0.94 [95% CI, 0.89–1.00]), MI (HR, 0.92 [95% CI, 0.81–1.04]), and IS (HR, 0.93 [95% CI, 0.84–1.03]), although this was not statistically significant.
Table 3.
Adjusted HRs of Cardiovascular Events Associated With Substituting 10 or 30 min of Activity for Another, Using Isotemporal Substitution Models
| HR (95% CI) | |||
|---|---|---|---|
| Total CVD | Myocardial infarction | Ischemic stroke | |
| Replace 10 min of SB with | |||
| MVPA | 0.96 (0.93–0.99) | 0.95 (0.89–1.01) | 0.96 (0.92–1.00) |
| HLPA | 1.02 (0.98–1.06) | 1.04 (0.95–1.13) | 1.03 (0.96–1.10) |
| Replace 30 min of SB with | |||
| MVPA | 0.88 (0.81–0.96) | 0.85 (0.71–1.02) | 0.88 (0.77–1.01) |
| HLPA | 1.05 (0.93–1.20) | 1.11 (0.85–1.45) | 1.10 (0.90–1.35) |
| Replace 10 min of HLPA with | |||
| MVPA | 0.94 (0.89–1.00) | 0.92 (0.81–1.04) | 0.93 (0.84–1.03) |
| Replace 30 min of HLPA with | |||
| MVPA | 0.84 (0.70–1.01) | 0.77 (0.52–1.13) | 0.80 (0.60–1.08) |
Cox proportional hazards models adjusted for confounders: age, self‐reported general health, postmenopausal hormone therapy, smoking status, and alcohol use. CVD indicates cardiovascular disease; HLPA, high‐light physical activity; HR, hazard ratio; MVPA, moderate to vigorous physical activity; and SB, sedentary behavior.
Effect Measure Modifiers
The associations of PA and SB with CVD outcomes were not modified by age, self‐reported general health, physical function, MVPA (for SB models only), or SB (for MVPA models only); see P values for interaction terms in Table S3. Among participants with obesity, being in the highest quartile of minutes per day of MVPA was not associated with lower hazards of total CVD (HR: 1.48 [95% CI: 0.80–2.74]) compared with the lowest quartile. Among participants without obesity, the greatest quartile of minutes per day of MVPA was associated with a lower hazard of total CVD (HR: 0.53 [95% CI: 0.38–0.74]) compared with the lowest quartile.
Sensitivity Analyses
After the removal of 353 participants who self‐reported fair/poor general health or who had cardiovascular events within the first year (total CVD: n=87, MI: n=17, IS: n=30), patterns of association from models 1 and 2 were similar although estimates were slightly attenuated and CIs were widened for analyses of all exposures and outcomes (data not shown).
DISCUSSION
In this population of women aged 62 to 89 years, greater daily total volume of PA and greater minutes per day of MVPA were associated with lower hazards of total CVD and IS. When represented as a continuous measure, greater daily total volume of PA and minutes per day of MVPA were suggestive of an association with lower MI incidence, but were not statistically significant. The replacement of any amount of SB with MVPA was associated with lower hazards of total CVD; associations of the replacement of 10 minutes of SB with MVPA were similar in magnitude and direction to those of the primary analyses not using substitution models. HLPA was not associated with any of the CVD outcomes, and the replacement of time spent in SB with HLPA was also not associated with lower hazards of any CVD outcomes. SB was positively associated with total CVD. These trends were robust in sensitivity analyses investigating bias from confounding attributable to prior health status. After further adjustment for potential mediators (eg, BMI, hypertension, high cholesterol, diabetes, physical function) estimates were largely attenuated and were no longer statistically significant, indicating that the associations we observed were likely mediated through these variables. Our results suggest that the association of minutes per day of MVPA with total CVD was modified by obesity, with a stronger inverse association among women without obesity compared with women with obesity; however, this may be a chance finding as there is little evidence of effect measure modification by BMI in prior literature. 11 , 15 , 18 , 20 , 26 Although this may be a chance finding, greater BMI is associated with increased CVD incidence, and the prevention of weight gain and treatment of obesity through PA may be beneficial in preventing CVD. 11 , 49
Our finding of an inverse association between total volume of PA and time to total CVD largely agrees with previously published studies with accelerometry measures, 14 , 15 , 16 , 19 , 20 , 22 , 23 as does the same finding for MVPA with time to total CVD. 14 , 15 , 16 , 18 , 19 , 20 , 22 , 23 , 26 However, some of these studies found more robust associations for total PA 19 , 20 , 22 or MVPA 14 , 15 , 20 , 22 with time to CVD when adjusting for confounders that may also be mediators, unlike the results of this study. Of these 5 studies, 2 adjusted for BMI, 19 , 22 2 for measures of high cholesterol, 14 , 19 and 4 for diabetes, 14 , 15 , 20 , 22 while all 5 adjusted for some measure of high blood pressure. This study included BMI, history of hypertension, history of high cholesterol, history of diabetes, and physical function as potential mediators in the fully adjusted models. The inclusion of all of these factors in combination may explain differing results from studies that found more robust results when adjusting for only select mediators. Disparate study results could also be attributable to differing populations and age; this study included only women 62 years and older.
Although HLPA was not associated with time to total CVD, MI, or IS in this population of women, previous studies have found conflicting evidence when examining total light PA. Greater daily minutes of accelerometer‐measured light PA was associated with lower CVD incidence in 5 prior studies, 14 , 17 , 18 , 19 , 23 while 4 found no association. 15 , 16 , 22 , 26 All studies that found an association between light PA and CVD incidence were conducted in similarly aged populations of older adults, while those finding no association were primarily among younger populations. 15 , 22 , 26 In this analysis, we chose to only assess the association of HLPA rather than total light PA (LLPA plus HLPA), because of the higher likelihood for SB to be misclassified as LLPA and vice versa, 6 and because previous studies in similarly aged populations only found associations between HLPA and CVD mortality, not LLPA. 23 Furthermore, the VM thresholds used to define PA intensity categories used in this study were calibrated in a population of older adults, appropriately resulting in lower threshold values of VM for each intensity category than in a younger population. 6 Other studies that did not use thresholds calibrated in an older population 14 may have misclassified MVPA as light PA for adults in older age groups, resulting in an association of light PA with CVD outcomes. The only other study among older adults that found no association between light PA and CVD incidence used thresholds for defining light PA that were calibrated in a population of older adults, 16 but some studies of older adults using age‐appropriate thresholds did find associations between light PA and CVD incidence. 17 , 18 , 19 , 23 These 2 reasons may partially explain why our results differ from some previous studies; however, more research is needed to determine the relationship between light PA and CVD risk.
Fewer daily minutes of SB were associated with lower hazards of total CVD in this population. Although self‐reported evidence on the association between SB and CVD is strong, 11 prior evidence has been mixed on the association of accelerometer‐measured SB with total CVD. Three studies found no association between total time spent in SB or SB accumulation patterns with CVD risk after adjustment for covariates. 14 , 22 , 28 Null findings may be due to inadequate power 28 or the use of uniaxial accelerometers, which may not be as precise in measuring SB. 15 In contrast, 5 other studies reported that greater daily sedentary time was associated with increased risk of total CVD. 15 , 16 , 18 , 26 , 27 Differing results may also be attributable to different definitions of total CVD, as some of these definitions of total CVD only include CVD events (MI, stroke, CVD death), while others include additional CVD diagnoses as well (eg, hypertensive diseases, pulmonary heart disease, atherosclerosis, and heart failure). In addition, some studies defined their CVD outcomes using International Classification of Diseases (ICD) codes, while others were confirmed by physician adjudication or medical record review, resulting in different sensitivities and specificities of outcome ascertainment methods. Other reasons for differences include whether bias from confounding attributable to prior health status was investigated in the analyses. 48
The current study is one of few to examine the relationships of accelerometer‐measured PA and SB with both time to MI and IS. Although MI and IS share many cardiovascular risk factors and are both commonly caused by atherosclerotic disease, few patients experience both MI and IS (≈<4% in general, with only 2 participants in our population experiencing both); therefore, we investigated time to MI and IS as separate outcomes. We found, although not statistically significant, a possible inverse linear association of total daily volume of PA and MVPA with time to MI in continuous analyses, but this association was less clear when exposures were represented as quartiles. SB was largely not associated with time to MI. Previous studies using self‐reported data have found an inverse association between PA and MI incidence, and have also reported a positive association for sedentary time. 50 , 51 , 52 To our knowledge, no studies to date have examined only MI as an outcome in relation to accelerometry‐measured PAs or SB. However, 4 studies examined coronary heart disease and ischemic heart disease, with 3 finding inverse associations with total PA and MVPA, 17 , 19 , 20 and 1 finding a positive association with SB. 27 These differing results may be attributable to the inclusion of non‐MI outcomes (coronary death, angina pectoris, and other acute and chronic ischemic heart diseases) 17 , 19 , 20 included in these other studies. Inclusion of more types of events may have contributed to an overall larger number of events and greater statistical power to detect effects, as MI in our population was less prevalent (0.7%) than coronary/ischemic heart disease in previous studies (2.4% to 4.9%), 17 , 19 , 20 , 27 or it may be that other events such as coronary death may be driving these results as opposed to MI.
In this cohort, greater daily total volume of PA and greater minutes per day of MVPA were associated with lower hazards of IS, but there was largely no association for SB. Only 3 studies investigated the relationship between accelerometer‐measured PAs with stroke, and one has explored the role of SB. Two studies found that total PA was inversely associated with incidence of stroke, 19 , 20 and 3 studies found the same association for MVPA. 19 , 20 , 29 Two studies found that greater levels of light PA were associated with decreased incidence of stroke, 19 , 29 and one found SB to be positively associated. 29 These studies defined stroke as either IS or hemorrhagic stroke, unlike our study, which only included IS. Varying definitions of stroke end points may contribute to conflicting findings for SB, as biological mechanisms may differ between the 2 types of stroke. 43
Through the use of isotemporal models, we found that replacing time spent in either SB or HLPA with MVPA was associated with lower hazards of total CVD. Point estimates were similar for MI and IS, but results were not statistically significant, and CIs were wide likely because of the small number of events. Replacing SB with HLPA was not associated with reduced hazards of any of the CVD outcomes. It may be that light PA does not derive as great of a benefit to cardiovascular health, as higher‐intensity aerobic activities may alter the cardiovascular system by improving capillary density, endothelial function, blood cholesterol levels, insulin sensitivity, BMI, stroke volume, blood coagulation, and peripheral resistance. 53 However, some studies have suggested that replacing SB with low‐intensity activities such as standing or stepping may provide similar, but not as numerous, benefits to the cardiovascular system such as lower BMI, blood pressure, blood glucose, and cholesterol levels. 54 Only one prior study investigated the hypothetical effect of replacing SB or light PA with MVPA on CVD incidence, with results similar to ours. 21 Studies that have explored CVD mortality as an outcome also found inverse associations of replacing 30 minutes of SB with light PA (HRs: 0.86 and 0.76, respectively). 24 , 25 However, these studies found that effect sizes were larger when replacing 30 minutes of SB with 30 minutes of MVPA (HRs: 0.35 and 0.23, respectively). 24 , 25 Further investigation is required to determine the effect of replacing SB with light PA, as plausible biological pathways between light PA and the cardiovascular system may exist.
Strengths and Limitations
Strengths of the current study include the large sample size and prospective design. Misclassification from measurement error in the exposure is less of a concern compared with self‐reports, attributable to device measurement of PA by accelerometry, and use of age‐appropriate intensity level cut points. We have additionally minimized the influence of measurement error in the exposure by only assessing HLPA, rather than total light PA. 6 This was one of the first studies to examine time to components of CVD, such as MI and IS, rather than only a “total CVD” outcome. No prior studies have investigated time to MI as a separate outcome with accelerometer‐measured activities. By assessing time to various CVD outcomes, this may help identify particular types of events that are driving overall associations with total CVD, and elucidate which biological mechanisms contribute most to the effect of PA and SB on cardiovascular health. The use of isotemporal models in this analysis is also a strength, as few studies have used these models.
Accelerometers are not able to assess strength training activities and have reduced accuracy at particularly slow walking speeds. This may have resulted in an underestimate of total PA and MVPA, leading to an overall underestimation of the effect size for these types of activities. In addition, statistical power was lost in analyses that separated the time to CVD outcomes into components (MI: n=108; IS: n=181), so these associations should be confirmed in future research. We were unable to investigate either time to cardiovascular death (n=29) or hemorrhagic stroke (n=22) as independent outcomes because of the small number of events. There is also the possibility of residual confounding from measurement error among confounders or unmeasured confounders.
The generalizability of these results may be limited. Accelerometer assessment was conditional on survival until this phase of the observational study, involvement in the cohort, and the ability to ambulate independently. These factors may all be related to PA and CVD risk, therefore minimizing generalizability of the results. We were also limited in that we could not assess differences in these associations by race and ethnicity, education level, or sex, as this is a fairly homogeneous population of older, female health care workers. Furthermore, this population is a relatively healthy and active population compared with populations of a similar age. 55 Future work should investigate these associations in younger, more diverse populations.
CONCLUSIONS
In this population of older women, greater total volume of PA and daily minutes of MVPA were associated with lower hazards of total CVD and IS. Greater daily minutes of SB were associated with greater hazards of total CVD but not MI or IS. HLPA was not associated with hazards of any CVD outcomes; more research is needed on whether HLPA should be included in guidelines for CVD risk reduction, as current literature displays conflicting results. For public health prevention of CVD, older women should be encouraged to replace time spent sedentary with MVPA.
Sources of Funding
Kennedy M. Peter‐Marske, Christopher Moore, and Carmen Cuthbertson were supported by a National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute National Research Service Award (T32‐HL007055). The study was supported by the NIH 5R01CA227122: National Cancer Institute, Office of the Director, Office of Disease Prevention, and Office of Behavioral and Social Sciences Research. WHS (Women's Health Study) is funded by NIH grants CA154647, CA047988, CA182913, HL043851, HL080467, and HL099355. Eric Shiroma was supported in part by the Intramural Research Program at the National Institute on Aging. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclosures
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supporting information
Tables S1–S3
Figure S1
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.028180
For Sources of Funding and Disclosures, see page 11.
References
- 1. Cardiovascular diseases (CVDs). World Health Organization. 2021. Accessed May 4, 2022. https://www.who.int/en/news‐room/fact‐sheets/detail/cardiovascular‐diseases‐(cvds)
- 2. Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore‐Mensah Y, et al. Heart disease and stroke statistics‐2022 update: a report from the American Heart Association. Circulation. 2022;145:e153–e639. doi: 10.1161/CIR.0000000000001052 [DOI] [PubMed] [Google Scholar]
- 3. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al. Heart disease and stroke statistics‐2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 4. Shiroma EJ, Lee IM. Physical activity and cardiovascular health: lessons learned from epidemiological studies across age, gender, and race/ethnicity. Circulation. 2010;122:743–752. doi: 10.1161/CIRCULATIONAHA.109.914721 [DOI] [PubMed] [Google Scholar]
- 5. Ekelund U, Tarp J, Steene‐Johannessen J, Hansen BH, Jefferis B, Fagerland MW, Whincup P, Diaz KM, Hooker SP, Chernofsky A, et al. Dose‐response associations between accelerometry measured physical activity and sedentary time and all cause mortality: systematic review and harmonised meta‐analysis. BMJ. 2019;366:l4570. doi: 10.1136/bmj.l4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Evenson KR, Wen F, Herring AH, Di C, LaMonte MJ, Tinker LF, Lee IM, Rillamas‐Sun E, LaCroix AZ, Buchner DM. Calibrating physical activity intensity for hip‐worn accelerometry in women age 60 to 91 years: the Women's Health Initiative OPACH Calibration Study. Prev Med Rep. 2015;2:750–756. doi: 10.1016/j.pmedr.2015.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hagstromer M, Troiano RP, Sjostrom M, Berrigan D. Levels and patterns of objectively assessed physical activity—a comparison between Sweden and the United States. Am J Epidemiol. 2010;171:1055–1064. doi: 10.1093/aje/kwq069 [DOI] [PubMed] [Google Scholar]
- 8. McPhee JS, French DP, Jackson D, Nazroo J, Pendleton N, Degens H. Physical activity in older age: perspectives for healthy ageing and frailty. Biogerontology. 2016;17:567–580. doi: 10.1007/s10522-016-9641-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chastin SFM, De Craemer M, De Cocker K, Powell L, Van Cauwenberg J, Dall P, Hamer M, Stamatakis E. How does light‐intensity physical activity associate with adult cardiometabolic health and mortality? Systematic review with meta‐analysis of experimental and observational studies. Br J Sports Med. 2019;53:370–376. doi: 10.1136/bjsports-2017-097563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Diaz KM, Howard VJ, Hutto B, Colabianchi N, Vena JE, Safford MM, Blair SN, Hooker SP. Patterns of sedentary behavior and mortality in U.S. middle‐aged and older adults: a national cohort study. Ann Intern Med. 2017;167:465–475. doi: 10.7326/M17-0212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. 2018 Physical Activity Guidelines Advisory Committee . 2018 Physical Activity Guidelines Advisory Committee Scientific Report. Washington, DC: U.S. Department of Health and Human Services; 2018. [Google Scholar]
- 12. Prince SA, Cardilli L, Reed JL, Saunders TJ, Kite C, Douillette K, Fournier K, Buckley JP. A comparison of self‐reported and device measured sedentary behaviour in adults: a systematic review and meta‐analysis. Int J Behav Nutr Phys Act. 2020;17:31. doi: 10.1186/s12966-020-00938-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sallis J, Saelens B. Assessment of physical activity by self‐report: status, limitations, and future directions. Res Q Exerc Sport. 2000;71:1–14. doi: 10.1080/02701367.2000.11082780 [DOI] [PubMed] [Google Scholar]
- 14. Dempsey PC, Strain T, Khaw KT, Wareham NJ, Brage S, Wijndaele K. Prospective associations of accelerometer‐measured physical activity and sedentary time with incident cardiovascular disease, cancer, and all‐cause mortality. Circulation. 2020;141:1113–1115. doi: 10.1161/CIRCULATIONAHA.119.043030 [DOI] [PubMed] [Google Scholar]
- 15. Dohrn IM, Welmer AK, Hagstromer M. Accelerometry‐assessed physical activity and sedentary time and associations with chronic disease and hospital visits—a prospective cohort study with 15 years follow‐up. Int J Behav Nutr Phys Act. 2019;16:125. doi: 10.1186/s12966-019-0878-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jefferis BJ, Parsons TJ, Sartini C, Ash S, Lennon LT, Papacosta O, Morris RW, Wannamethee SG, Lee IM, Whincup PH. Does total volume of physical activity matter more than pattern for onset of CVD? A prospective cohort study of older British men. Int J Cardiol. 2019;278:267–272. doi: 10.1016/j.ijcard.2018.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. LaCroix AZ, Bellettiere J, Rillamas‐Sun E, Di C, Evenson KR, Lewis CE, Buchner DM, Stefanick ML, Lee IM, Rosenberg DE, et al. Association of light physical activity measured by accelerometry and incidence of coronary heart disease and cardiovascular disease in older women. JAMA Netw Open. 2019;2:e190419. doi: 10.1001/jamanetworkopen.2019.0419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ensrud KE, Blackwell TL, Cauley JA, Dam TTL, Cawthon PM, Schousboe JT, Barrett‐Connor E, Stone KL, Bauer DC, Shikany JM, et al. Objective measures of activity level and mortality in older men. J Am Geriatr Soc. 2014;62:2079–2087. doi: 10.1111/jgs.13101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nguyen S, Bellettiere J, Wang G, Di C, Natarajan L, LaMonte MJ, LaCroix AZ. Accelerometer‐derived daily life movement classified by machine learning and incidence of cardiovascular disease in older women: the OPACH study. J Am Heart Assoc. 2022;11:e023433. doi: 10.1161/JAHA.121.023433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ramakrishnan R, Doherty A, Smith‐Byrne K, Rahimi K, Bennett D, Woodward M, Walmsley R, Dwyer T. Accelerometer measured physical activity and the incidence of cardiovascular disease: evidence from the UK Biobank cohort study. PLoS Med. 2021;18:e1003487. doi: 10.1371/journal.pmed.1003487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yerramalla MS, McGregor DE, van Hees VT, Fayosse A, Dugravot A, Tabak AG, Chen M, Chastin SFM, Sabia S. Association of daily composition of physical activity and sedentary behaviour with incidence of cardiovascular disease in older adults. Int J Behav Nutr Phys Act. 2021;18:83. doi: 10.1186/s12966-021-01157-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Evenson KR, Wen F, Herring AH. Associations of accelerometry‐assessed and self‐reported physical activity and sedentary behavior with all‐cause and cardiovascular mortality among US adults. Am J Epidemiol. 2016;184:621–632. doi: 10.1093/aje/kww070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. LaMonte MJ, Buchner DM, Rillamas‐Sun E, Di C, Evenson KR, Bellettiere J, Lewis CE, Lee IM, Tinker LF, Seguin R, et al. Accelerometer‐measured physical activity and mortality in women aged 63 to 99. J Am Geriatr Soc. 2018;66:886–894. doi: 10.1111/jgs.15201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schmid D, Ricci C, Baumeister SE, Leitzmann MF. Replacing sedentary time with physical activity in relation to mortality. Med Sci Sports Exerc. 2016;48:1312–1319. doi: 10.1249/MSS.0000000000000913 [DOI] [PubMed] [Google Scholar]
- 25. Dohrn IM, Kwak L, Oja P, Sjostrom M, Hagstromer M. Replacing sedentary time with physical activity: a 15‐year follow‐up of mortality in a national cohort. Clin Epidemiol. 2018;10:179–186. doi: 10.2147/CLEP.S151613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dohrn IM, Sjostrom M, Kwak L, Oja P, Hagstromer M. Accelerometer‐measured sedentary time and physical activity‐a 15 year follow‐up of mortality in a Swedish population‐based cohort. J Sci Med Sport. 2018;21:702–707. doi: 10.1016/j.jsams.2017.10.035 [DOI] [PubMed] [Google Scholar]
- 27. Bellettiere J, LaMonte MJ, Evenson KR, Rillamas‐Sun E, Kerr J, Lee IM, Di C, Rosenberg DE, Stefanick M, Buchner DM, et al. Sedentary behavior and cardiovascular disease in older women: the Objective Physical Activity and Cardiovascular Health (OPACH) study. Circulation. 2019;139:1036–1046. doi: 10.1161/CIRCULATIONAHA.118.035312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dempsey PC, Strain T, Winkler EAH, Westgate K, Rennie KL, Wareham NJ, Brage S, Wijndaele K. Association of accelerometer‐measured sedentary accumulation patterns with incident cardiovascular disease, cancer, and all‐cause mortality. J Am Heart Assoc. 2022;11:e023845. doi: 10.1161/JAHA.121.023845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hooker SP, Diaz KM, Blair SN, Colabianchi N, Hutto B, McDonnell MN, Vena JE, Howard VJ. Association of accelerometer‐measured sedentary time and physical activity with risk of stroke among US adults. JAMA Network Open. 2022;5. doi: 10.1001/jamanetworkopen.2022.15385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cook NR, Lee IM, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH, Buring JE. Low‐dose aspirin in the primary prevention of cancer the Women's Health Study: a randomized controlled trial. J Am Med Assoc. 2005;294:47–55. doi: 10.1001/jama.294.1.47 [DOI] [PubMed] [Google Scholar]
- 31. Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH, Buring JE. Vitamin E and the primary prevention of cardiovascular disease and cancer the Women's Health Study: a randomized controlled trial. J Am Med Assoc. 2005;294:56–65. doi: 10.1001/jama.294.1.56 [DOI] [PubMed] [Google Scholar]
- 32. Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, Hennekens CH, Buring JE. A randomized trial of low‐dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–1304. doi: 10.1056/NEJMoa050613 [DOI] [PubMed] [Google Scholar]
- 33. Lee IM, Shiroma EJ, Evenson KR, Kamada M, LaCroix AZ, Buring JE. Using devices to assess physical activity and sedentary behavior in a large cohort study, the Women's Health Study. J Meas Phys Behav. 2018;1:60–69. doi: 10.1123/jmpb.2018-0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tudor‐Locke C, Camhi SM, Troiano RP. A catalog of rules, variables, and definitions applied to accelerometer data in the National Health and Nutrition Examination Survey, 2003–2006. Prev Chronic Dis. 2012;9:E113. doi: 10.5888/pcd9.110332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Keadle SK, Shiroma EJ, Freedson PS, Lee IM. Impact of accelerometer data processing decisions on the sample size, wear time and physical activity level of a large cohort study. BMC Public Health. 2014;14. doi: 10.1186/1471-2458-14-1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Choi L, Ward SC, Schnelle JF, Buchowski MS. Assessment of wear/nonwear time classification algorithms for triaxial accelerometer. Med Sci Sports Exerc. 2012;44:2009–2016. doi: 10.1249/MSS.0b013e318258cb36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shiroma EJ, Kamada M, Smith C, Harris TB, Lee IM. Visual inspection for determining days when accelerometer is worn: is this valid? Med Sci Sports Exerc. 2015;47:2558–2562. doi: 10.1249/MSS.0000000000000725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Choi L, Liu Z, Matthews CE, Buchowski MS. Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sports Exerc. 2011;43:357–364. doi: 10.1249/MSS.0b013e3181ed61a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD; The Writing Group on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60:1581–1595. doi: 10.1016/j.jacc.2012.08.001 [DOI] [PubMed] [Google Scholar]
- 40. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE III. Classification of subtype of acute ischemic stroke definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.STR.24.1.35 [DOI] [PubMed] [Google Scholar]
- 41. Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, Gibson H, Gordon D, Copeland T, D'Agostino D, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380:33–44. doi: 10.1056/NEJMoa1809944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Putaala J, Nieminen T. Stroke risk period after acute myocardial infarction revised. J Am Heart Assoc. 2018;7:e011200. doi: 10.1161/JAHA.118.011200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Alevizos A, Lentzas J, Kokkoris S, Mariolis A, Korantzopoulos P. Physical activity and stroke risk. Int J Clin Pract. 2005;59:922–930. doi: 10.1111/j.1742-1241.2005.00536.x [DOI] [PubMed] [Google Scholar]
- 44. Hays RD, Donald Sherbourne C, Mazel RM. The RAND 36‐Item Health Survey 1.0. Health Econ. 1993;2:217–227. doi: 10.1002/hec.4730020305 [DOI] [PubMed] [Google Scholar]
- 45. Matthews CE, Keadle SK, Troiano RP, Kahle L, Koster A, Brychta R, Van Domelen D, Caserotti P, Chen KY, Harris TB, et al. Accelerometer‐measured dose‐response for physical activity, sedentary time, and mortality in US adults. Am J Clin Nutr. 2016;104:1424–1432. doi: 10.3945/ajcn.116.135129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Desquilbet L, Mariotti F. Dose‐response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29:1037–1057. doi: 10.1002/sim.3841 [DOI] [PubMed] [Google Scholar]
- 47. Mekary RA, Willett WC, Hu FB, Ding EL. Isotemporal substitution paradigm for physical activity epidemiology and weight change. Am J Epidemiol. 2009;170:519–527. doi: 10.1093/aje/kwp163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Matthews CE, Troiano RP, Salerno EA, Berrigan D, Patel SB, Shiroma EJ, Saint‐Maurice PF. Exploration of confounding due to poor health in an accelerometer‐mortality study. Med Sci Sports Exerc. 2020;52:2546–2553. doi: 10.1249/MSS.0000000000002405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Powell‐Wiley TM, Poirier P, Burke LE, Despres JP, Gordon‐Larsen P, Lavie CJ, Lear SA, Ndumele CE, Neeland IJ, Sanders P, et al. Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2021;143:e984–e1010. doi: 10.1161/CIR.0000000000000973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hummel M, Hantikainen E, Adami HO, Ye W, Bellocco R, Bonn SE, Lagerros YT. Association between total and leisure time physical activity and risk of myocardial infarction and stroke—a Swedish cohort study. BMC Public Health. 2022;22:532. doi: 10.1186/s12889-022-12923-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Oliveira A, Barros H, Azevedo A, Bastos J, Lopes C. Impact of risk factors for non‐fatal acute myocardial infarction. Eur J Epidemiol. 2009;24:425–432. doi: 10.1007/s10654-009-9352-9 [DOI] [PubMed] [Google Scholar]
- 52. Held C, Iqbal R, Lear SA, Rosengren A, Islam S, Mathew J, Yusuf S. Physical activity levels, ownership of goods promoting sedentary behaviour and risk of myocardial infarction: results of the INTERHEART study. Eur Heart J. 2012;33:452–466. doi: 10.1093/eurheartj/ehr432 [DOI] [PubMed] [Google Scholar]
- 53. Powell KE, Paluch AE, Blair SN. Physical activity for health: What kind? How much? How intense? On top of what? Annu Rev Public Health. 2011;32:349–365. doi: 10.1146/annurev-publhealth-031210-101151 [DOI] [PubMed] [Google Scholar]
- 54. Healy GN, Winkler EAH, Owen N, Anuradha S, Dunstan DW. Replacing sitting time with standing or stepping: associations with cardio‐metabolic risk biomarkers. Eur Heart J. 2015;36:2643–2649. doi: 10.1093/eurheartj/ehv308 [DOI] [PubMed] [Google Scholar]
- 55. Evenson KR, Bellettiere J, Cuthbertson CC, Di C, Dushkes R, Howard AG, Parada H Jr, Schumacher BT, Shiroma EJ, Wang G, et al. Cohort profile: the Women's Health Accelerometry Collaboration. BMJ Open. 2021;11:e052038. doi: 10.1136/bmjopen-2021-052038 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3
Figure S1


