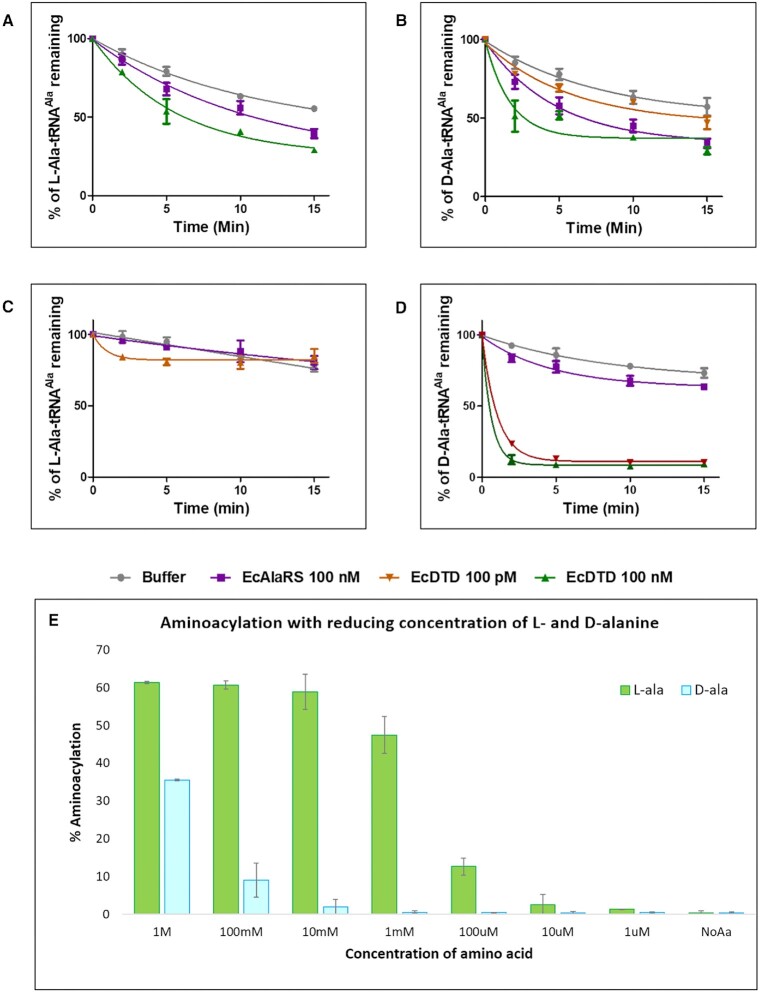
Figure 5.
Chiral selectivity of AlaRS aminoacylation site. (A) Deacylation of L-Ala-tRNAAla by both EcDTD and EcAlaRS-WT. (B) Deacylation of D-Ala-tRNAAla (AlaRS generated aminoacylated substrate) by EcAlaRS-WT and EcDTD showing that D-Ala-tRNAAla is not cleaved by either of them. (C) Deacylation of L-Ala-tRNAAla charged by flexizyme by EcAlaRS-WT and EcDTD showing similar results as AlaRS charged substrate. (D) Deacylation of flexizyme charged D-Ala-tRNAAla by EcAlaRS-WT and EcDTD shows that EcDTD can effectively cleave D-Ala-tRNAAla but not by EcAlaRS-WT. (E) Aminoacylation of L-Ala and D-Ala on tRNAAla by EcAlaRS C666A with reducing concentration of the amino acid substrate in the reaction (100 mM, 10 mM, 1 mM, 100 μM, 10 μM, 1 μM and 100 nM) showing L-alanine getting charged at 10 μM amino acid concentration, which is 1000-fold less than the minimum concentration at which D-alanine charging is seen, i.e. 10 mM.