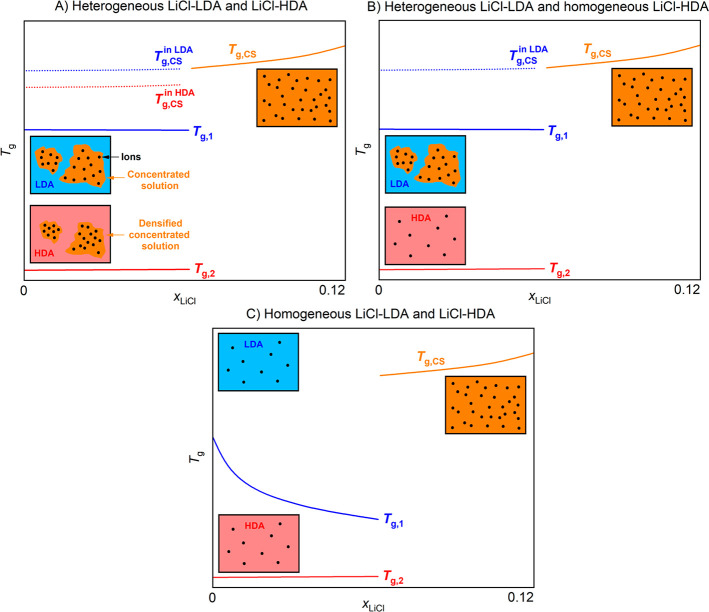
Figure 11.
Three possible scenarios regarding the behavior of glassy LiClaq and the hypothesized effects on the glass transition temperatures based on literature and our results. (A) Both LiCl-LDA and LiCl-HDA are heterogeneous and phase separate into a water-rich part (LDA or HDA) and a CS. At low concentrations, this results in the appearance of the glass transitions of pure HDA Tg,2 or LDA Tg,1, along with the glass transition of the separated CS in the HDA (Tg,CSin HDA) or LDA (Tg,CSin LDA) matrix. At high concentrations (≥5.8 mol %, R = 16.2), only the glass transition of the CS Tg,CS is observed. (B) LiCl is only insoluble in LDA but not in HDA. Therefore, HDA is homogeneous and separates into LDA and a CS in the course of the polyamorphic transition. In contrast to the scenario described in panel (A), no Tg,CSin HDA is observed. (C) LiCl-LDA and LiCl-HDA are fully homogeneous. Below 5.8 mol %, either Tg,2 (LiCl-HDA) or Tg,1 (LiCl-LDA) appears, whereas above 5.8 mol %, neither LiCl-HDA nor LiCL-LDA are observed but a homogeneous CS is formed instead. Only the Tg,CS value is found at ∼140 K, so that a jumplike change in Tg is found at ∼5 mol %.