Abstract
Animal model systems are dependent on the standardization of husbandry protocols that maximize growth and reduce generation time. The Mexican tetra, Astyanax mexicanus, exists as eyed surface and blind cave dwelling populations. The opportunity for comparative approaches between independently evolved populations has led to the rapid growth of A. mexicanus as a model for evolution and biomedical research. However, a slow and inconsistent growth rate remains a major limitation to the expanded application of A. mexicanus. Fortunately, this temporal limitation can be addressed through husbandry changes that accelerate growth rates while maintaining optimal health outcomes. Here, we describe a husbandry protocol that produces rapid growth rates through changes in diet, feeding frequency, growth sorting and progressive changes in tank size. This protocol produced robust growth rates and decreased the age of sexual maturity in comparison to our previous protocol. To determine whether changes in feeding impacted behavior, we tested fish in exploration and schooling assays. We found no difference in behavior between the two groups, suggesting that increased feeding and rapid growth will not impact the natural variation in behavioral traits. Taken together, this standardized husbandry protocol will accelerate the development of A. mexicanus as a genetic model.
Keywords: Astyanax mexicanus, cavefish, husbandry, evolution, behavioral neuroscience
Introduction
Husbandry for animal models has improved greatly over the past century, due to the scientific community's desire to keep healthy stocks that create reproducible data.1–3 Over time, changes in the size of tanks and housing for laboratory animals,4–6 improved diets7–9 and environmental enrichment6,10–12 have provided healthier breeding stocks, while also boosting growth rates. A majority of genetic and biomedical research is performed in a small number of models, including Caenorhabditis elegans, Drosophila, zebrafish, and mice. A recognized commonality between these models is ease of husbandry, relatively fast generation times, and rapid growth rates under standardized conditions.1,4,5,8,10,13 These traits, along with optimized husbandry protocols have facilitated major discoveries and widespread use of these models.
As genome sequencing, along with mutagenesis and transgene technologies have become less expensive, non-traditional laboratory organisms are starting to be adopted world-wide.14–18 Further, the innovation of modern genetic tools, including gene editing, have allowed for the expanded use of models to address diverse biological questions. Increasingly, these new models are being used to address the evolution of complex traits, that are largely intractable in classic genetic models. Many of these non-traditional models have major impediments that need to be addressed, such as challenges in laboratory rearing15–17 due to slow generation times, a loss of natural environmental cues, or a general lack of published data on husbandry practices.
Aquatic organisms such as teleost fish provide advantages for studying the biology of vertebrates.18–22 Fish models provide large clutch sizes that are easy to collect and maintain. Many taxa have embryonic and larval stage-fish that are transparent, allowing researchers to study internal organs without euthanizing, dissecting or disturbing tissue.20,22–24 The blind Mexican tetra (Astyanax mexicanus) is a teleost species that is emerging in the fields of evolution, development, and neuroscience.25–27
The species is found in two distinct forms: an eyed, surface-dwelling form, and several hydrologically isolated populations of cavefish. Cavefish populations are the result of ancestral surface fish being washed into caves between 100,000 and 300,000 years ago,28 resulting in the convergence of traits, such as eye degeneration29,30 and loss of pigment,31,32 or loss of sleep22,33 and decreased stress.34 Although cavefish have evolved troglomorphic phenotypes, surface to cave hybrids are viable and can be crossed for allele segregation and subsequent quantitative trait loci mapping.35–37
Fish husbandry in the A. mexicanus community continues to vary across fish facilities and research groups.14,26,38 The recent implementation of gene editing39,40 and Tol2-based transgenesis,14,41 along with the consistent breeding of surface × cave hybrid populations for genetic mapping,29,31,32,37 would benefit from shorter generation times38,42,43 without jeopardizing survival for reared fish. Although our initial goal was to standardize feeding, because we utilized variables tested in previous animal models,4,7–9,44 such as a high-nutrient diet, lower tank densities, and feeding/tank size scaling with body length, we also optimized growth rates and maximized animal welfare.
To define a standard protocol for optimizing growth rates, we used bimonthly observational and management periods to determine whether changes in diet, feeding frequency, and tank conditions across growth improved rearing times. Our new husbandry protocol implemented several changes that include: scaling food particle size to match caloric density to fish size, reducing tank densities (fish/L), and sorting fish according to standard length (SL) to match food type and tank size. In our experience, these changes in husbandry resulted in a temporal reduction to reach sexual maturity, from 8 to 10 months to 5 months post-fertilization.
Finally, because diet can impact animal behavior, we tested whether this change in diet altered preexisting behavioral phenotypes for surface and cave populations. Fish raised under our new protocol showed no difference in individual or group behavior. These results provide a clear rationale for adopting this protocol for A. mexicanus husbandry; high growth rates, lower mortality, and shorter generation time, with no observable impact on natural behavioral variation.
Materials and Methods
Fish maintenance and husbandry
A. mexicanus were cared for in accordance with NIH guidelines, and all experiments were approved by the Florida Atlantic University Institutional Care and Use Committee Protocol #A1929. A. mexicanus stocks were housed in the Florida Atlantic Universities Mexican tetra core facilities. A. mexicanus fish lines used for this study; Pachón cavefish stocks were initially derived from Richard Borowsky (NYU); Surface fish stocks were derived from Rio Choy wild populations. Both populations were raised and maintained at 23–24°C, pH 7.9–8.2, 621–764 μS of conductance, 1.3–1.9 mg/L, and a 14:10 L:D light cycle.
Dietary ingredients and feeding schedule
Control fish stocks were fed using Tetra® TetraMin Tropical Flakes (Spectrum Brannds Pet, LLC., Blacksburg, VA, USA) with a nutritional content of 40% crude protein, 5% crude fat, 5% crude fiber, 9% moisture, and 9% ash. Fish fed under our new protocol changed diet during development; Brine shrimp—60% protein, 24% fat, 4.4% ash, and 8.5% moisture—was used to feed week-old larvae and as a supplement at early stages.
Gemma (GEMMA micro 150; Skretting, Inc., Westbrook, ME, USA) as larvae, ground blood worms (Hikari Bio-Pure, blood worms; Kyorin Food Industries, Ltd., Tokyo, Japan) and Zeigler pellets as juveniles (Zeigler Bros., Inc., Gardners, PA, USA), and blood worms and Zeigler pellets as adults. Gemma (100–500 pellet size) nutritional content; 59% protein, 14% oil, 14% ash, 0.2% fiber, and 1.3% phosphorus. Zeigler pellets nutritional content; 45% protein, 16% fat, 2% fiber, 12% moisture, and 8% ash. Fish were fed to satiation, and tank cleaning was performed every other week to promote excellent water quality and high oxygenation.
SL measurements
Videos were collected with rulers placed at the front and back of each tank. Frames were analyzed in Fiji using the line segment and measurement tools to record SL. Scale was coded using the rulers in each picture by using the line segment and set scale tools. Five fish per tank were measured for each time point and continued measuring through 50 mm in SL. An SL of 50 mm was chosen as a target, because previous laboratory members have reported successful breeding of 45–55 mm SL adults.
Novel tank assay
Adult fish were transported to a dedicated behavioral room to acclimate for 1 h. After acclimation, individual fish were added singly to 2.4 L of water in 2.8 L plastic zebrafish tanks (Aquaneering, Inc., San Diego, CA, USA) and recorded using an FLIR Grasshopper®3, GS3-U3-23S6M-C 1/1.2 (Teledyne FLIR LLC., Wilsonville, OR, USA) at 15 fps for 15 min. Videos were then analyzed using Noldus EthoVision® Software XT14 (Noldus, Inc., Wageningen, Netherlands) to track and measure time spent at the top and bottom half of the tank.
Schooling assay
Fish were carefully transferred to the behavioral room, gently netted into the experimental arena, and allowed to acclimate for 10 min. Experiments were conducted in a round tank (111 cm diameter × 66 cm height) filled to a depth of 9 cm with system water. A Genius WideCam F100 video camera (Dongguan Gaoying Computer Products Co., Guangdong, China) was affixed to a custom-built PVC stand that allowed recording from above the center of the tank.
Lighting was provided via four white 75-W equivalent halogen light bulbs (Philips A19 Long Life Light Bulb, Amsterdam, Netherlands) mounted in clamp lights with 5.5 in shades (HDX; The Home Depot, Georgia, USA) to diffuse light. Videos were collected at 30 fps using OBS Studio (Open Broadcaster Software).
Automated tracking was done using EthoVision XT v. 13.0.1220, and raw data were used to calculate median distance between pairs of fish and median centroid speed using the Pandas and NumPy libraries in Python 3.9.5. Pair distance was calculated as the distance between the center points of each fish, and centroid speed was calculated as the movement speed of the center point directly between individuals. The Shapiro-Wilk test was performed to assess normality of pair distance and centroid speed medians. Data were subsequently compared using unpaired t-tests. All statistical analyses were performed using GraphPad Prism 9.2.1.
Results
Scaling nutrient density of food source with SL provides high growth rates and fast generation times
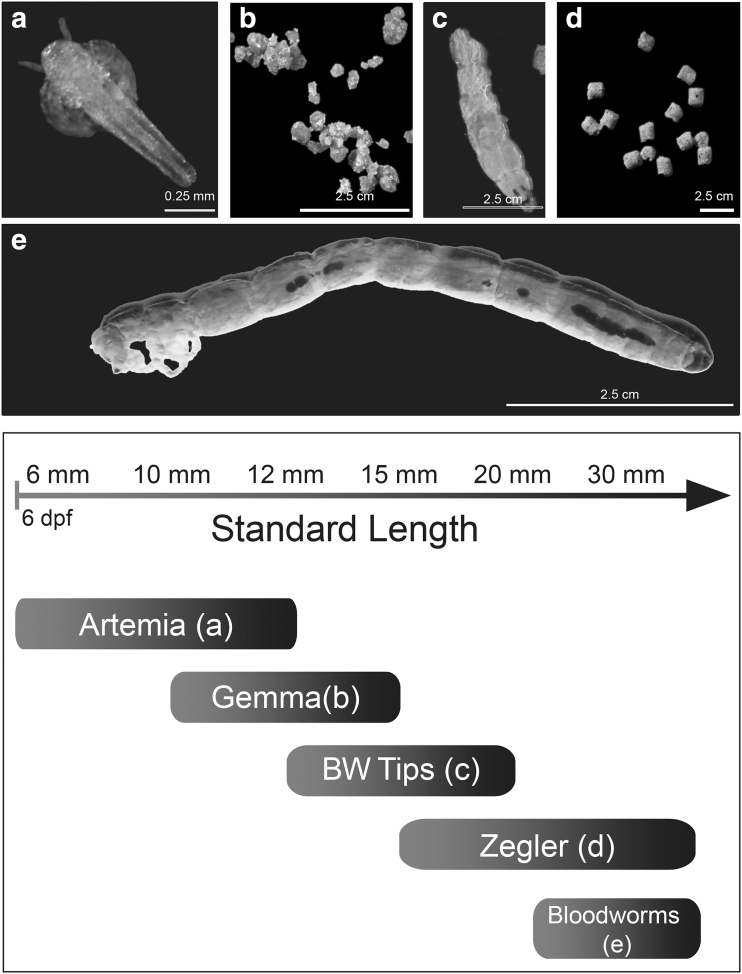
Nutrient uptake is limited by the size of the mouth and digestive track during larval and juvenile periods, limiting growth during early development.7,45 To provide a standardized diet, we increased feeding frequency and food particle size of high protein content feed across life stages, decreasing feeding effort while increasing nutritional value (Table 1). Larvae were initially fed freshly hatched artemia shrimp once daily after 6 days post-fertilization (dpf) (Fig. 1 and Table 1).
Table 1.
Feeding Schedule Matching Standard Length to Feeding Type and Frequency
| Fish size in SL | Feed types (frequency) |
|---|---|
| 6–10 mm | Artemia (once) |
| >10 mm | Artemia (once), Gemma 300 μm (once) |
| >12 mm | Gemma 300 μm (once), blood worm segment tips (once) |
| >15 mm | Ground Zeigler pellets (twice), blood worm segment tips (once) |
| >20 mm | Zeigler pellets (twice), blood worms (twice) |
| >30 mm | Zeigler pellets (thrice), blood worms (thrice) |
SL = length of a straight line from snout to the end of the caudal peduncle.
SL, standard length.
FIG. 1.
Food type and feeding schedule for our novel high growth rate protocol. (a) Twenty-four hours hatched artemia nauplius. (b) Gemma pellet feed. (c) Blood worm tips. (d) Zeigler pellets. (e) Full blood worm. Arrow denotes fish size in SL (mm; distance from nose to caudal peduncle). SL, standard length.
By the time fish had a length equal to 10 mm (4–6 weeks), 300 μm Gemma (Fig. 1b and Table 1) was added once a day as a supplement to hatched artemia. Once larvae exceeded 12 mm SL, brine shrimp was removed and blood worm segment tips were then added as a supplement (Fig. 1c and Table 1) and animals were monitored during feeding to ensure no fry struggled with ingestion. Studies have shown that fish prefer bloodworms in comparison to other feed, providing a dietary enrichment that promotes feeding and growth.46,47
At 15 mm (8–10 weeks), feeding was changed from Gemma to ground Zeigler pellets twice daily (Fig. 1d) and blood worms (Fig. 1e and Table 1) once a day. Zeigler pellets provide an improvement in nutrient density and decrease feeding time, due to particle size (Gemma = 300 μM, Ground Zeigler = ∼1 mm). At 20 mm SL, fish were switched to full Zeigler pellets (3 mm diameter) and blood worms twice daily. Finally, at 30 mm SL, feeding was increased to morning, afternoon, and evening.
For example, at 9 AM fish would consume Zeigler pellets within a 5-min time-span, followed by a feeding of blood worms. This feeding schedule was then followed through the end of the study, along with breeding protocols that were performed every 2 weeks. Fertilization of healthy embryos was recorded at 42–50 mm SL (∼4–5 months), achieving high growth rates and a reduction in time to sexual maturity.
Sorting fish by SL and creating a scalable tank size schedule results in low variation of growth across populations
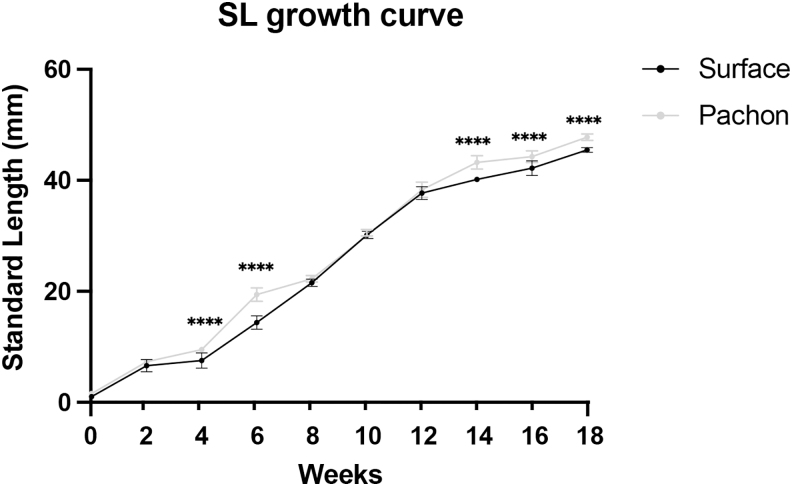
Tank densities (fish/L) have been shown to impact the growth and health of developing fish.48–50 To ensure robust growth rates and optimal health outcomes, we created a schedule for increasing tank size based on SL. SL was measured at bimonthly time points, beginning at 2 weeks of development, before the fish were put onto the aquarium system (Tables 2 and 3; Fig. 2). Cavefish aged 4–6 weeks had a larger SL in comparison to surface fish (Table 2 and Fig. 2; Supplementary Tables S1 and S2).
Tables 2.
Standard Length (Standard Length) in mm Across 20 Weeks of Development
| 2 | 4 | 6 | 8 | 10 | 12 | 14 | 16 | 18 | 20 | |
|---|---|---|---|---|---|---|---|---|---|---|
| SF | 7.5 ± 0.6 | 12.6 ± 1.0 | 13.5 ± 1.3 | 19.7 ± 1.1 | 26.3 ± 0.8 | 34.1 ± 0.7 | 41.0 ± 1.0 | 43.3 ± 0.9 | 45.1 ± 1.2 | 48.1 ± 0.7 |
| C | 8.0 ± 0.6 | 13.3 ± 0.8 | 15.3 ± 0.6 | 24.3 ± 1.1 | 26.9 ± 0.7 | 34.0 ± 0.8 | 41.6 ± 1.2 | 46.1 ± 1.1 | 47.0 ± 1.0 | 50.2 ± 0.7 |
± standard deviation.
C, cavefish; SF, surface fish.
Table 3.
Schedule for Changing Tank Sizes Across Development
| Fish size in SL (mm) | Tank size |
| 7–14 Days post-fertilization | 12 cm diameter glass bowl |
| 6–12 mm | 2.8 L plastic tank |
| 12–20 mm | 9 L zebrafish tank |
| 20 − 40 mm | 5 gal |
| >40 mm | 10 gal |
SL, standard length.
FIG. 2.
SL measurements of surface fish and Pachón cavefish through 5 months of development. SL measurements in millimeters (y-axis) were recoded every 2 weeks (x-axis) until fish reached 50 mm in SL. x-axis starts at week 2 when fish were measured and put onto the recirculating system in the fish facility. Error bars denote ±standard error. Sample size, n = 24 for both surface fish and cavefish. Unpaired t-test with significance values; ****p < 0.0001.
Fish were then progressively sorted and placed in larger tanks (Table 3; 9-L and 5-gal). When their SL was 40 mm, fish were finally moved to 10-gallon tanks. Fish exhibited robust growth rates throughout the tank schedule changes, from week 4–6 and week 14–18, with average SL increasing nearly fourfold from week 6 to 14 (Table 2 and Fig. 2). These results provide a standard for high growth rates through the coupling of tank size and SL.
High protein meals that are stage matched for feeding frequency reduce mortality rates and improve adult welfare
We monitored mortality and tank density throughout development to determine what stages contributed to mortality, and whether increasing the frequency of feeding impacted health and tank behavior. Larvae were added to the fish facility system at a density of 16 fish per tank (2.8 L). Mortality rates for fry during the first month averaged 22% across populations, resulting in a tank density of 10–13 fish per tank (average = 12 ± 0.22 fish; Table 4).
Table 4.
Tank Population Size and Mortality Rate Recorded at the Final Week of the Study
| Tank 1 | Tank 2 | Tank 3 | Tank 4 | Tank 5 | Average | |
|---|---|---|---|---|---|---|
| SF | 13 (0.13) | 11 (0.26) | 12 (0.20) | 12 (0.20) | 11 (0.26) | 11.8 (0.22) |
| C | 12 (0.20) | 13 (0.13) | 10 (0.33) | 11 (0.26) | 12 (0.20) | 11.6 (0.23) |
(No.) = mortality rate of tank.
Following the first month of development, we did not record further mortality in any tank for all populations (Table 4). In addition to low mortality rates, we also observed few instances of surface fish exhibiting attacking behavior (reviewing all video taken for growth recording and observations made by the husbandry staff). Further, throughout the duration of the study, only one fish needed to be isolated due to aggression of siblings. Overall, our new feeding strategy, tank size, and sorting scheduling produced low mortality rates and lower aggression.
Exploration behavior and schooling in adult surface fish and cavefish populations are not impacted by changes in husbandry practices
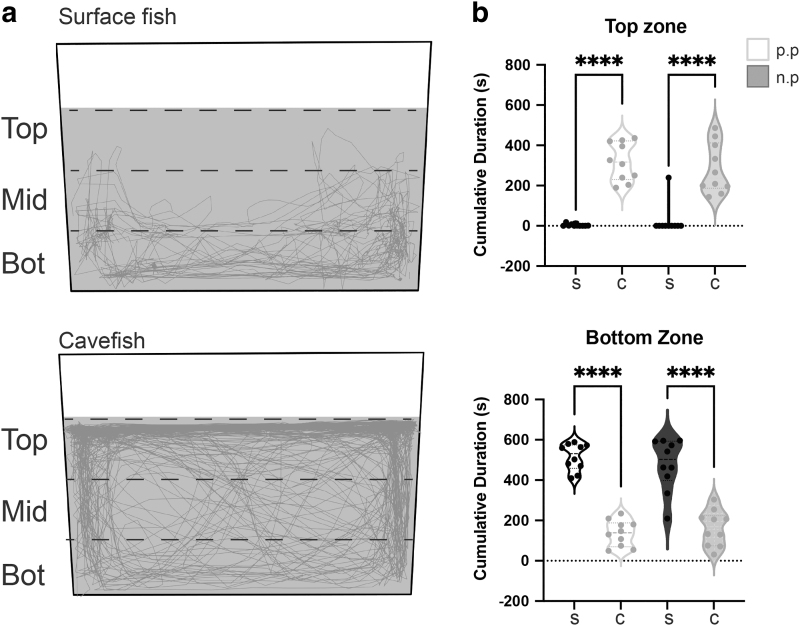
Changes to animal care can impact animal behavior and therefore influence the reproducibility of behavioral studies.10,51,52 To determine whether our changes in animal care could impact behavior, we utilized standard protocols for published behavioral assays at the individual and group level.31,53 Behavioral and physiological indicators of stress are reduced in cavefish compared with surface fish.34 The novel tank assay measures stress response from determining the ratio of time spent in the top (low stress) versus bottom (high stress) zones of the tank.34
Our results recapitulate previously published results, with surface fish displaying high stress (bottom dwelling) and Pachòn cavefish exhibiting low stress (top dwelling) across husbandry protocols (Fig. 3; Supplementary Tables S3–S6). Finally, to determine how group behavior is impacted by our new protocol, pairs of adult A. mexicanus were observed for schooling behavior in a 111 cm diameter tank for 20 min.
FIG. 3.
Novel tank assay comparing adult Astyanax populations raised with previous and high growth rate husbandry protocol. (a) Tracking traces (grey) from individual novel tank trials, with gray shading representing water column. (b) Cumulative duration of time spent in the top and bottom half of the tank. Previous husbandry protocol (p.p.) and new husbandry protocol (n.p.). Violin plots display minimum to maximum values, with three lines representing the 75th quartile, median, and 25th quartile. Sample size (n = 10) were the same for all populations. ****p < 0.0001.
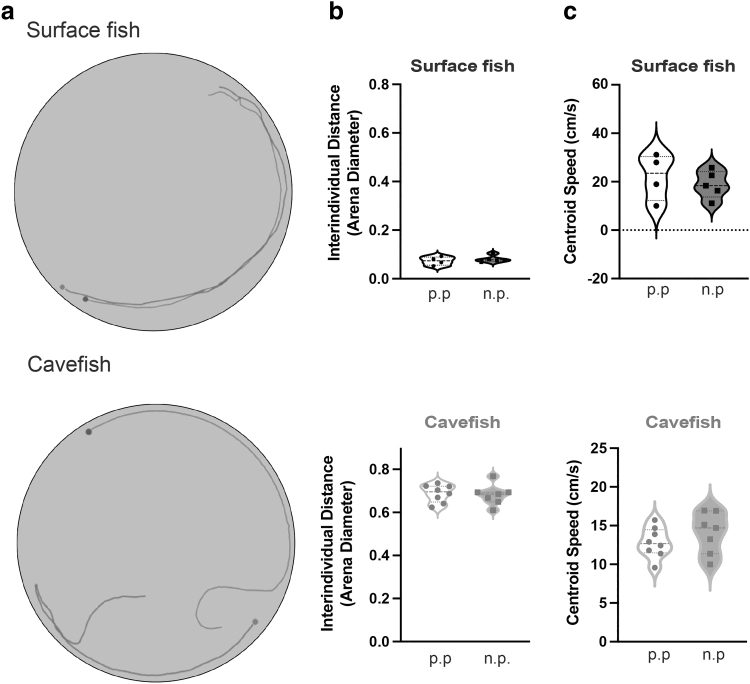
Again, fish raised with our new standard protocol recapitulated previously published data,53 with Pachón cavefish showing no evidence of attraction, exhibiting large interindividual distances when raised under both old and new rearing protocols, while surface fish display schooling behaviors, exhibiting short interindividual distances when raised under both old and new rearing protocols (Fig. 4; Supplementary Tables S7–S10). These results suggest that our changes to husbandry do not impact well-established individual and group behaviors of surface fish and cavefish populations. Therefore, these protocols increase the growth and survival of this model without impacting complex behaviors commonly studied in this system.
FIG. 4.
Schooling assay comparing fish from adult Astyanax populations raised with previous and high growth rate husbandry protocol. (a) Swimming tracks representing a single trial of two fish from the same population (e.g., grey = fish 1, black = fish 2). (b) Violin plots of interindividual distance, the average distance in cm between both fish in each trial, normalized to arena diameter (111 cm). (c) Violin plots of centroid speed, average speed calculated from tracking a centroid placed at the middle of each fish. Previous husbandry protocol (p.p.) and new husbandry protocol (n.p.). Violin plots display minimum to maximum values, with three lines representing the 75th quartile, median, and 25th quartile. Sample sizes for surface fish, previous n = 4 groups, and new husbandry protocol n = 5 groups. Sample sizes for cavefish fish, previous n = 8 groups, and new husbandry n = 7 groups. No comparisons were statistically significant.
Discussion
A standardized husbandry protocol is necessary as the cavefish community grows across the globe. Cavefish provide a model with high-genetic diversity for direct genotype-phenotype research in relation to evolution and disease. However, the low growth rates26,42,43 and larger tank sizes needed for cavefish husbandry in comparison to other aquatic models26,38 make the establishment of new generations more costly in terms of time and fish capacity. Here, we show that a progression in particle size of high nutrient feed, tank density, tank size, and uniform SL sorting provides a high growth rate that can result in healthy breeding adults by 5 months post-fertilization.
This protocol will greatly decrease the time it takes to develop pure and hybrids lines, along with stable transgenics and CRISPR-induced mutants. With the growth and health outcomes achieved, coupled with experiments showing that a change in diet did not affect behavior, we suggest that cavefish community members improve their adult generation timetable by adopting this husbandry protocol.
Enriched early life diet and increased feeding frequency resulted in accelerated growth
Past laboratory studies have commented on food sources for A. mexicanus, but they vary in the types of feed and feeding frequencies.14,38,54 We previously fed populations of A. mexicanus artemia for larval and juvenile periods, switching to flake food twice daily for adults. Therefore, we decided to utilize a typical zebrafish feeding regime, increasing particle size as the fry grow, scaling appropriately with jaw and stomach size.41 In this study, we went from feeding artemia, to small grain Gemma, before scaling up to feeding Zeigler pellets and blood worms.
We also decided to omit flake food, which loses its nutrient value while sitting in the water column.55 In the zebrafish community, 2–3 feedings per day is recommended because fish lack a true stomach and therefore food boluses pass through quickly allowing for increased feeding.56–58 Our goal was to raise 50 mm SL adults that could produce healthy embryos. This goal was achieved with a robust growth period (week 6–18), which likely represents a critical period of development for reaching a larger size, along with sexual maturity in a reasonable timetable.
Changes in diet, tank density, and tank size for SL improved survival rates and appeared to lower aggression in surface fish tanks
Importantly, we observed a low mortality rate during development and overall decreased aggression in adult surface fish populations. In this study, all mortality occurred within the first month of development, a period of slow growth before fish display a robust growth rate. Further, a combined survival rate of 78% is higher than previously published rates of 18% for surface fish and 36% for cavefish,14 and mirrors the high survival rates observed in recent zebrafish husbandry methods.38,59 Also in the current study, we did not record a single mortality after 6 weeks of development. We find that an average tank density of 2–3 fish per gallon results in steady growth and good health outcomes for developing fish.
In addition, smaller fish can be chased and bit by aggressors in adult populations, which can result in either bodily injury or death to the fish being attacked.60 Surprisingly, we only observed one incident that resulted in the transfer of a fish for isolation and recuperation. Lack of fighting/biting injuries was later confirmed by reviewing images and videos taken for length measurements, revealing no observable injuries to surface fish across growth periods. These results suggest that, not only is our revised protocol ideal for quickly raising adults, but it also improves community health by lowering aggression.
No observed behavioral change in fish raised with optimized protocol
Several zebrafish studies have shown that changes in diet and environment can affect a fish's physiology and behavior.61–63 Due to the importance of reproducibility in scientific research, it is paramount that biological data are consistent across labs and do not vary due to changes in diet and husbandry practices. When comparing our standard to optimized protocol reared fish, we found no change in adult exploration or schooling behavior of either surface fish or cavefish populations.
One recent zebrafish study showed that lower feeding frequency increased anxiety in adult zebrafish, which may help explain the reduction in aggressive behaviors that we observed with more feeding.63,64 Certainly, an increase in tank size, density, and an additional feeding period per day, could provide enough resources across 24 h to reduce competition. Overall, this study provides a standardized way to increase growth rates and decrease time to sexual maturity, while still maintaining population specific phenotypes that are necessary for studying the evolution of complex traits.
Conclusion
Our results provide a husbandry protocol that facilitates robust growth rates and health outcomes for A. mexicanus populations. This is likely to greatly improve existing applications and experiments, such as adult fish research, replenishment of breeding stocks, and generating hundreds of hybrid fish that are necessary for Quantitative Trait Loci mapping. Our findings clearly show that a change in diet and management of populations across growth periods can drastically reduce the time it takes to raise fish to adulthood, while also improving mortality and maintaining a healthy behavioral environment.
Supplementary Material
Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by NSF EDGE grant 1923372 (E.R.D. and J.E.K.), NSF grant IOS2202359 (J.E.K.), NIH grant R35GM138345 (J.E.K.), NIH grant R15HD099022 (J.E.K.), and NIH grant R24OD030214 (A.C.K.).
Supplementary Material
References
- 1. Alestrom P, D'Angelo L, Midtlyng PJ, et al. . Zebrafish: Housing and husbandry recommendations. Lab Anim 2020;54(3):213–224; doi: 10.1177/0023677219869037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lawrence C, Eisen JS, Varga ZM. Husbandry and Health Program Survey synopsis. Zebrafish 2016;13 Suppl 1:S5–S7; doi: 10.1089/zeb.2016.1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Varga ZM. Aquaculture, husbandry, and shipping at the Zebrafish International Resource Center. Methods Cell Biol 2016;135:509–534; doi: 10.1016/bs.mcb.2016.01.007 [DOI] [PubMed] [Google Scholar]
- 4. Lawrence C. Advances in zebrafish husbandry and management. Methods Cell Biol 2011;104:429–451; doi: 10.1016/B978-0-12-374814-0.00023-9 [DOI] [PubMed] [Google Scholar]
- 5. Gonder JC, Laber K. A renewed look at laboratory rodent housing and management. ILAR J 2007;48(1):29–36; doi: 10.1093/ilar.48.1.29 [DOI] [PubMed] [Google Scholar]
- 6. Toth LA, Trammell RA, Ilsley-Woods M. Interactions between housing density and ambient temperature in the Cage Environment: Effects on mouse physiology and behavior. J Am Assoc Lab Anim Sci 2015;54(6):708–717. [PMC free article] [PubMed] [Google Scholar]
- 7. Watts SA, Lawrence C, Powell M, et al. . The vital relationship between nutrition and health in Zebrafish. Zebrafish 2016;13 Suppl 1:S72–S76; doi: 10.1089/zeb.2016.1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jennings M, Batchelor GR, Brain PF, et al. . Refining rodent husbandry: The mouse. Report of the Rodent Refinement Working Party. Lab Anim 1998;32(3):233–259; doi: 10.1258/002367798780559301 [DOI] [PubMed] [Google Scholar]
- 9. Hawkins P, Morton DB, Bevan R, et al. . Husbandry refinements for rats, mice, dogs and non-human primates used in telemetry procedures. Seventh report of the BVAAWF/FRAME/RSPCA/UFAW Joint Working Group on Refinement, Part B. Lab Anim 2004;38(1):1–10; doi: 10.1258/00236770460734335 [DOI] [PubMed] [Google Scholar]
- 10. Toth LA. The influence of the cage environment on rodent physiology and behavior: Implications for reproducibility of pre-clinical rodent research. Exp Neurol 2015;270:72–77; doi: 10.1016/j.expneurol.2015.04.010 [DOI] [PubMed] [Google Scholar]
- 11. Corcoran M. Environmental enrichment for aquatic animals. Vet Clin North Am Exot Anim Pract 2015;18(2):305–321; doi: 10.1016/j.cvex.2015.01.004 [DOI] [PubMed] [Google Scholar]
- 12. Feng Y, Fan H, Liang X, et al. . Environmental enrichment changes rabbits' behavior, serum hormone level and further affects cecal microbiota. PeerJ 2022;10:e13068; doi: 10.7717/peerj.13068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Corsi AK, Wightman B, Chalfie M. A transparent window into biology: A primer on Caenorhabditis elegans (vol 200, pg 387, 2015). Genetics 2015;201(1):339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Elipot Y, Legendre L, Pere S, et al. . Astyanax transgenesis and husbandry: How cavefish enters the laboratory. Zebrafish 2014;11(4):291–299; doi: 10.1089/zeb.2014.1005 [DOI] [PubMed] [Google Scholar]
- 15. Juntti S. The future of gene-guided neuroscience research in non-traditional model organisms. Brain Behav Evol 2019;93(2–3):108–121 [DOI] [PubMed] [Google Scholar]
- 16. Ke Z, Vaidya A, Ascher J, et al. . Novel husbandry techniques support survival of naked mole rat (Heterocephalus glaber) pups. J Am Assoc Lab Anim Sci 2014;53(1):89–91. [PMC free article] [PubMed] [Google Scholar]
- 17. Presnell JS, Bubel M, Knowles T, et al. . Multigenerational laboratory culture of pelagic ctenophores and CRISPR-Cas9 genome editing in the lobate Mnemiopsis leidyi. Nat Protoc 2022;17(8):1868–1900. [DOI] [PubMed] [Google Scholar]
- 18. Powder KE, Albertson RC. Cichlid fishes as a model to understand normal and clinical craniofacial variation. Dev Biol 2016;415(2):338–346; doi: 10.1016/j.ydbio.2015.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bassar RD, Childs DZ, Rees M, et al. . The effects of asymmetric competition on the life history of Trinidadian guppies. Ecol Lett 2016;19(3):268–278; doi: 10.1111/ele.12563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schulze L, Henninger J, Kadobianskyi M, et al. . Transparent Danionella translucida as a genetically tractable vertebrate brain model. Nat Methods 2018;15(11):977–983; doi: 10.1038/s41592-018-0144-6 [DOI] [PubMed] [Google Scholar]
- 21. Heng K, Thompson A, Chu D, et al. . Three cheers for the three-spined stickleback. Lab Anim (N Y) 2016;45(11):421; doi: 10.1038/laban.1142 [DOI] [PubMed] [Google Scholar]
- 22. Keene AC, Duboue ER. The origins and evolution of sleep. J Exp Biol 2018;221(Pt 11); doi: 10.1242/jeb.159533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kozol RA, Abrams AJ, James DM, et al. . Function over form: Modeling groups of inherited neurological conditions in Zebrafish. Front Mol Neurosci 2016;9:55; doi: 10.3389/fnmol.2016.00055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen E, Ekker SC. Zebrafish as a genomics research model. Curr Pharm Biotechnol 2004;5(5):409–413; doi: 10.2174/1389201043376652 [DOI] [PubMed] [Google Scholar]
- 25. McGaugh SE, Kowalko JE, Duboue E, et al. . Dark world rises: The emergence of cavefish as a model for the study of evolution, development, behavior, and disease. J Exp Zool B Mol Dev Evol 2020;334(7–8):397–404; doi: 10.1002/jez.b.22978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hinaux H, Pottin K, Chalhoub H, et al. . A developmental staging table for Astyanax mexicanus surface fish and Pachon cavefish. Zebrafish 2011;8(4):155–165; doi: 10.1089/zeb.2011.0713 [DOI] [PubMed] [Google Scholar]
- 27. Jeffery WR. Cavefish as a model system in evolutionary developmental biology. Dev Biol 2001;231(1):1–12; doi: 10.1006/dbio.2000.0121 [DOI] [PubMed] [Google Scholar]
- 28. Herman A, Brandvain Y, Weagley J, et al. . The role of gene flow in rapid and repeated evolution of cave-related traits in Mexican tetra, Astyanax mexicanus. Mol Ecol 2018;27(22):4397–4416; doi: 10.1111/mec.14877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yamamoto Y, Stock DW, Jeffery WR. Hedgehog signalling controls eye degeneration in blind cavefish. Nature 2004;431(7010):844–847; doi: 10.1038/nature02864 [DOI] [PubMed] [Google Scholar]
- 30. Sifuentes-Romero I, Ferrufino E, Thakur S, et al. . Repeated evolution of eye loss in Mexican cavefish: Evidence of similar developmental mechanisms in independently evolved populations. J Exp Zool B Mol Dev Evol 2020;334(7–8):423–437; doi: 10.1002/jez.b.22977 [DOI] [PubMed] [Google Scholar]
- 31. O'Gorman M, Thakur S, Imrie G, et al. . Pleiotropic function of the oca2 gene underlies the evolution of sleep loss and albinism in cavefish. Curr Biol 2021;31(16):3694–3701.e4; doi: 10.1016/j.cub.2021.06.077 [DOI] [PubMed] [Google Scholar]
- 32. Protas ME, Hersey C, Kochanek D, et al. . Genetic analysis of cavefish reveals molecular convergence in the evolution of albinism. Nat Genet 2006;38(1):107–111; doi: 10.1038/ng1700 [DOI] [PubMed] [Google Scholar]
- 33. Duboue ER, Keene AC, Borowsky RL. Evolutionary convergence on sleep loss in cavefish populations. Curr Biol 2011;21(8):671–676; doi: 10.1016/j.cub.2011.03.020 [DOI] [PubMed] [Google Scholar]
- 34. Chin JSR, Gassant CE, Amaral PM, et al. . Convergence on reduced stress behavior in the Mexican blind cavefish. Dev Biol 2018;441(2):319–327; doi: 10.1016/j.ydbio.2018.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kowalko J. Utilizing the blind cavefish Astyanax mexicanus to understand the genetic basis of behavioral evolution. J Exp Biol 2020;223(Pt Suppl 1); doi: 10.1242/jeb.208835 [DOI] [PubMed] [Google Scholar]
- 36. Warren WC, Boggs TE, Borowsky R, et al. . A chromosome-level genome of Astyanax mexicanus surface fish for comparing population-specific genetic differences contributing to trait evolution. Nat Commun 2021;12(1):1447; doi: 10.1038/s41467-021-21733-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kowalko JE, Rohner N, Linden TA, et al. . Convergence in feeding posture occurs through different genetic loci in independently evolved cave populations of Astyanax mexicanus. Proc Natl Acad Sci U S A 2013;110(42):16933–16938; doi: 10.1073/pnas.1317192110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Baumann DP, Ingalls A. Mexican tetra (Astyanax mexicanus): Biology, Husbandry, and Experimental Protocols. In: Laboratory Fish in Biomedical Research. (D'Angelo L, de Girolamo P. eds.) Academic Press: Cambridge, MA; 2022; pp. 311–347. [Google Scholar]
- 39. Ma L, Jeffery WR, Essner JJ, et al. . Genome editing using TALENs in blind Mexican Cavefish, Astyanax mexicanus. PLoS One 2015;10(3):e0119370; doi: 10.1371/journal.pone.0119370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Klaassen H, Wang Y, Adamski K, et al. . CRISPR mutagenesis confirms the role of oca2 in melanin pigmentation in Astyanax mexicanus. Dev Biol 2018;441(2):313–318; doi: 10.1016/j.ydbio.2018.03.014 [DOI] [PubMed] [Google Scholar]
- 41. Stahl BA, Peuss R, McDole B, et al. . Stable transgenesis in Astyanax mexicanus using the Tol2 transposase system. Dev Dyn 2019;248(8):679–687; doi: 10.1002/dvdy.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tabin JA, Aspiras A, Martineau B, et al. . Temperature preference of cave and surface populations of Astyanax mexicanus. Dev Biol 2018;441(2):338–344; doi: 10.1016/j.ydbio.2018.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Simon V, Elleboode R, Mahe K, et al. . Comparing growth in surface and cave morphs of the species Astyanax mexicanus: Insights from scales. Evodevo 2017;8:23; doi: 10.1186/s13227-017-0086-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Norton A, Franse KF, Daw T, et al. . Larval rearing methods for small-scale production of healthy zebrafish. East Biol 2019;2019(Spec Issue):33–46. [PMC free article] [PubMed] [Google Scholar]
- 45. Ronnestad I, Yufera M, Ueberschar B, et al. . Feeding behaviour and digestive physiology in larval fish: Current knowledge, and gaps and bottlenecks in research. Rev Aquacult 2013;5:S59–S98; doi: 10.1111/raq.12010 [DOI] [Google Scholar]
- 46. Fard MS, Pasmans F, Adriaensen C, et al. . Chironomidae bloodworms larvae as aquatic amphibian food. Zoo Biol 2014;33(3):221–227; doi: 10.1002/zoo.21122 [DOI] [PubMed] [Google Scholar]
- 47. Volkman ET, Pangle KL, Rajchel DA, . Hatchery performance attributes of juvenile lake sturgeon fed two natural food types. N Am J Aquacult 2004;66(2):105–112; doi: 10.1577/A03-047.1 [DOI] [Google Scholar]
- 48. Sahoo SK, Giri SS, Sahu AK. Effect of stocking density on growth and survival of Clarias batrachus (Linn.) larvae and fry during hatchery rearing. J Appl Ichthyol 2004;20(4):302–305; doi: 10.1111/j.1439-0426.2004.00534.x [DOI] [Google Scholar]
- 49. Alvarez-Gonzalez CA, Ortiz-Galindo JL, Dumas S, et al. . Effect of stocking density on the growth and survival of spotted sand bass Paralabrax maculatofasciatus larvae in a closed recirculating system. J World Aquacult Soc 2001;32(1):130–137; doi: 10.1111/j.1749-7345.2001.tb00932.x [DOI] [Google Scholar]
- 50. El-Sayed AFM. Effects of stocking density and feeding levels on growth and feed efficiency of Nile tilapia (Oreochromis niloticus L.) fry. Aquac Res 2002;33(8):621–626; doi: 10.1046/j.1365-2109.2002.00700.x [DOI] [Google Scholar]
- 51. Huntingford FA. Implications of domestication and rearing conditions for the behaviour of cultivated fishes. J Fish Biol 2004;65:122–142; doi: 10.1111/j.0022-1112.2004.00562.x [DOI] [Google Scholar]
- 52. Andersson M, Kettunen P. Effects of holding density on the Welfare of Zebrafish: A systematic review. Zebrafish 2021;18(5):297–306; doi: 10.1089/zeb.2021.0018 [DOI] [PubMed] [Google Scholar]
- 53. Kowalko JE, Rohner N, Rompani SB, et al. . Loss of schooling behavior in cavefish through sight-dependent and sight-independent mechanisms. Curr Biol 2013;23(19):1874–1883; doi: 10.1016/j.cub.2013.07.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Borowsky R. Breeding Astyanax mexicanus Through Natural Spawning. Cold Spring Harbor Protocols: Long Island, NY; 2008. [DOI] [PubMed] [Google Scholar]
- 55. Gomez-Requeni P, Conceicao LE, Olderbakk Jordal AE, et al. . A reference growth curve for nutritional experiments in zebrafish (Danio rerio) and changes in whole body proteome during development. Fish Physiol Biochem 2010;36(4):1199–1215; doi: 10.1007/s10695-010-9400-0 [DOI] [PubMed] [Google Scholar]
- 56. Francis-Floyd R. Fish Nutrition: University of Florida Cooperative Extenstion Service, Institute of Food and Agricultural Sciences, EDIS: Gainesville, FL; 2002. [Google Scholar]
- 57. Farmer SC. Chapter 24—Introduction to Zebrafish Husbandry. Academic Press; 2019; p. 1019. [Google Scholar]
- 58. Wang Z, Du J, Lam SH, et al. . Morphological and molecular evidence for functional organization along the rostrocaudal axis of the adult zebrafish intestine. BMC Genomics 2010;11:392; doi: 10.1186/1471-2164-11-392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hernandez RE, Galitan L, Cameron J, et al. . Delay of initial feeding of zebrafish larvae until 8 days postfertilization has no impact on survival or growth through the Juvenile Stage. Zebrafish 2018;15(5):515–518; doi: 10.1089/zeb.2018.1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Petty BD, Francis-Floyd R. Pet fish care and husbandry. Vet Clin North Am Exot Anim Pract 2004;7(2):397–419, vii; doi: 10.1016/j.cvex.2004.02.003 [DOI] [PubMed] [Google Scholar]
- 61. Stevens CH, Reed BT, Hawkins P. Enrichment for Laboratory Zebrafish—A review of the evidence and the challenges. Animals (Basel) 2021;11(3):698; doi: 10.3390/ani11030698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lieggi C, Kalueff AV, Lawrence C, et al. . The influence of behavioral, social, and environmental factors on reproducibility and replicability in Aquatic Animal Models. ILAR J 2020;60(2):270–288; doi: 10.1093/ilar/ilz019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dametto FS, Fior D, Idalencio R, et al. . Feeding regimen modulates zebrafish behavior. PeerJ 2018;6:e5343; doi: 10.7717/peerj.5343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rodriguez-Morales R, Gonzalez-Lerma P, Yuiska A, et al. . Convergence on reduced aggression through shared behavioral traits in multiple populations of Astyanax mexicanus. BMC Ecol Evol 2022;22(1):116; doi: 10.1186/s12862-022-02069-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.