Abstract
Background:
Bottled water (BW) consumption in the United States and globally has increased amidst heightened concern about environmental contaminant exposures and health risks in drinking water supplies, despite a paucity of directly comparable, environmentally-relevant contaminant exposure data for BW. This study provides insight into exposures and cumulative risks to human health from inorganic/organic/microbial contaminants in BW.
Methods:
BW from 30 total domestic US (23) and imported (7) sources, including purified tapwater (7) and spring water (23), were analyzed for 3 field parameters, 53 inorganics, 465 organics, 14 microbial metrics, and in vitro estrogen receptor (ER) bioactivity. Health-benchmark-weighted cumulative hazard indices and ratios of organic-contaminant in vitro exposure-activity cutoffs were assessed for detected regulated and unregulated inorganic and organic contaminants.
Results:
48 inorganics and 45 organics were detected in sampled BW. No enforceable chemical quality standards were exceeded, but several inorganic and organic contaminants with maximum contaminant level goal(s) (MCLG) of zero (no known safe level of exposure to vulnerable sub-populations) were detected. Among these, arsenic, lead, and uranium were detected in 67 %, 17 %, and 57 % of BW, respectively, almost exclusively in spring-sourced samples not treated by advanced filtration. Organic MCLG exceedances included frequent detections of disinfection byproducts (DBP) in tapwater-sourced BW and sporadic detections of DBP and volatile organic chemicals in BW sourced from tapwater and springs. Precautionary health-based screening levels were exceeded frequently and attributed primarily to DBP in tapwater-sourced BW and co-occurring inorganic and organic contaminants in spring-sourced BW.
Conclusion:
The results indicate that simultaneous exposures to multiple drinking-water contaminants of potential human-health concern are common in BW. Improved understandings of human exposures based on more environmentally realistic and directly comparable point-of-use exposure characterizations, like this BW study, are essential to public health because drinking water is a biological necessity and, consequently, a high-vulnerability vector for human contaminant exposures.
Keywords: Bottled water, Contaminant mixtures, Organics, Inorganics, Microorganisms, Human health
1. Introduction
The quality and long-term sustainability of drinking water (drinking/cooking water, collectively) are societal priorities and increasing concerns in the United States (US) (Allaire et al. 2018; Javidi and Pierce 2018; Pierce and Gonzalez 2017) and worldwide (Doria, 2010; Tröger et al. 2018; Villanueva et al. 2014), due to, among other reasons, population-driven increases in water use/reuse demands (DeSimone et al. 2015; Dieter et al. 2018) and in anthropogenic source-water contamination (Bexfield et al. 2019; Bexfield et al. 2021; DeSimone et al. 2015; Toccalino et al. 2012). In the US and globally, drinking water is delivered to consumers via three general supply chains or distribution “pipelines” (public tapwater [TW], private TW, bottled water [BW]), each with distinct logistical, infrastructure, regulatory, and commercial profiles, but all similarly challenged by an increasingly anthropized water cycle. Many water-borne pathogens and contaminants are actively regulated and monitored in US public-supply TW under the Safe Drinking Water Act (SDWA) (U.S. Environmental Protection Agency 2021a, e) and in BW as a food under the Food, Drugs, and Cosmetics Act (FD&C Act) (U.S. Food & Drug Administration 2021) and corresponding amendments; private–supply TW, however, is not systematically regulated or monitored (U.S. Environmental Protection Agency 2021b). Despite these regulatory differences, anthropogenic (i.e., human-–generated or –driven) contaminant concerns are common to all three pipelines because existing drinking-water regulations (Health Canada 2020; U.S. Environmental Protection Agency 2021a,e; World Health Organization (WHO) 2011) do not encompass many of the anthropogenic chemicals reported in ambient surface-water or groundwater source waters (Bradley et al. 2017; de Jesus Gaffney et al. 2015; DeSimone et al. 2015; Toccalino et al. 2012), much less the hundreds of thousands of synthetic chemicals estimated to be in commercial use globally (Wang et al. 2020).
The United States Geological Survey (USGS) collaborates with the Environmental Protection Agency (EPA), Food and Drug Administration (FDA), National Cancer Institute (NCI), National Institute of Allergy and Infectious Disease (NIAID), National Institute of Environmental Health Science (NIEHS), tribal nations, universities, water utilities, communities, and others to inform drinking-water exposure and water-supply data gaps by assessing inorganic/organic/microbial contaminant mixtures in point–of–use (POU) drinking water (Bradley et al. 2018; Bradley et al. 2020; Bradley et al. 2021a; Bradley et al. 2021b). Sampling personnel, collection protocols, core target-analytical methods and laboratories, and quality assurance/quality control procedures are maintained to ensure direct comparability across study areas and drinking-water distribution pipelines. Studies to date have focused on assessing contaminant mixtures in private– and public–supply TW and their associated distal (e.g., ambient source water) and proximal (e.g., premise plumbing, POU treatment) drivers in a range of socioeconomic and source-water vulnerability settings across the US. In 2020, USGS, FDA, EPA, and NIEHS conducted a reconnaissance of simultaneous inorganic/organic/microbial exposures in a cross–section (30 total) of individual-serving BW available in the US. This study was initiated to provide insight into cumulative contaminant risk (Moretto et al. 2017; National Research Council 1983; Norton et al. 1992) to human health from contaminants in BW and to expand the national perspective on inorganic/organic/microbial contaminant exposures in POU drinking-water by maintaining the approach employed across the US in previous POU TW studies by this group (Bradley et al. 2018; Bradley et al. 2020; Bradley et al. 2021a; Bradley et al. 2021b; Bradley et al. 2022).
For this study, BW exposure was operationally represented as detections (concentrations) of 53 inorganic and 465 unique organic analytes, 14 microbial metrics, and 1 in vitro bioactivity in BW samples. Potential human-health risks of individual and aggregate TW exposures were explored based on two lines of evidence: 1) cumulative detections and concentrations of designed-bioactive (e.g., pesticides, pharmaceuticals) chemicals (Bradley et al., 2017; Bradley et al. 2018; Bradley et al. 2020) and 2) exposure metrics based on cumulative Exposure-Activity Ratio(s) (ΣEAR) (Blackwell et al. 2017) and hazard indices (HI, cumulative toxicity/hazard quotients for mixtures (Goumenou and Tsatsakis 2019; U.S. Environmental Protection Agency 2011, 2012)) of cumulative benchmark-based Toxicity Quotients (ΣTQ) (Corsi et al. 2019).
Multiple TW-exposure hypotheses, relevant to BW specifically and to POU drinking water in general, were assessed. In line with an increasingly anthropized water cycle and with previous TW results by this research group (Bradley et al. 2018; Bradley et al. 2020; Bradley et al. 2021a; Bradley et al. 2021b; Bradley et al. 2022) and others (e.g., de Jesus Gaffney et al. 2015; Focazio et al. 2006; Knobeloch et al. 2013; Postma et al. 2011; Rogan and Brady 2009), simultaneous exposures to multiple inorganic and organic constituents of potential human-health interest were expected to occur in BW samples (Hypothesis I). Exceedances of FDA-enforceable BW standard of quality (SOQ, “shall not contain in excess of”) levels (U.S. Food & Drug Administration 2021); adopted from and, with few exceptions (e.g., lead [Pb]), equivalent to EPA public-supply enforceable National Primary Drinking Water Regulation maximum contaminant level(s) (MCL) (U.S. Environmental Protection Agency 2021a, e); were not expected (Hypothesis II). However, exceedances of EPA MCL goal(s) (MCLG, maximum level of a contaminant in drinking water at which no known or anticipated adverse effect on the health of sensitive subpopulations would occur, allowing an adequate margin of safety) (U.S. Environmental Protection Agency 2021d), other non-enforceable health-only advisories, or stricter state-promulgated enforceable standards were expected to occur in some BW samples (Hypothesis III).
2. Methods
2.1. Source selection and sampling
For this reconnaissance of potential human exposures to an expanded range of inorganic, organic, and microbial contaminants in BW, a cross-section of 30 BW brands (anonymized) available commercially in the US were selected to cover a variety of 1) source locations (US domestic, imported), 2) source types (spring or artesian [referred to collectively as “spring”], “purified” public-supply TW [purified-TW]), 3) purification treatments, and 4) packaging materials (glass, aluminum, carton) (Table S1) and analyzed one time each. For organic–chemical analyses and bioassays, samples were prepared by pouring water from the original BW packaging into the appropriate analytical sample bottle at the USGS New Jersey Water Science Center laboratory and were then shipped on ice overnight to the respective analytical laboratory. Controls for sampling artifacts (nominal field blanks) were prepared in the same manner and location using reagent blank waters. For inorganic–chemical and microbial analyses, BW samples were delivered in their original packaging to the analytical laboratory for processing and analysis.
2.2. Analytical methods
Briefly, BW samples were analyzed by USGS using 5 inorganic (53 analytes), 8 target-organic (465 unique analytes), 3 field parameter, and 14 microbial methods (Table S2), as discussed (Bradley et al. 2018; Bradley et al. 2020; Bradley et al. 2021a; Romanok et al. 2018) and described in detail previously (American Public Health Association 2018a,b,c,d; Ball and McCleskey 2003; Barringer and Johnsson 1996; Cohn et al. 1968; Furlong et al. 2014; Graham et al. 2010; Hajna 1955; Hergenreder 2011; Hladik et al. 2014; Hoffman et al. 1996; Kolpin et al. 2021; Kozak et al. 2013; Levin and Cabelli 1972; Lilley and Brewer 1953; Lisle and Priscu 2004; Loftin et al. 2016; McCleskey et al. 2019; Petrisek and Hall 2017; Rose et al. 2016; Sandstrom et al., 2015; U.S. Environmental Protection Agency 1997, 2014; U.S. Geological Survey variously dated). Pharmaceutical and pesticide samples were syringe filtered (0.7 μm nominal pore size, glass fiber) prior to analysis (Furlong et al. 2014; Sandstrom et al., 2015). The T47D-KBluc (American Type Cell Culture, ATCC, Manassas, VA; #CRL-2865) estrogen receptor transcriptional activation bioassay, previously developed (Wilson et al. 2004) and applied to environmental samples (Conley et al. 2017a; Medlock Kakaley et al. 2020) and treated tapwater (Conley et al. 2017b; Medlock Kakaley et al. 2021) was used to screen bottled water extracts for estrogenic activity (Neale et al. 2021). Cell culture maintenance and bioassay were performed as previously described (Wilson et al. 2004) with exceptions (Medlock Kakaley et al. 2020). Cells were exposed to a 17β-estradiol (E2; CAS #: 50-28-2; purity 98 %; catalog no. E887; lot: 28H0818) standard curve (0, 0.3, 1.0, 3.0, 10, and 30 pM), ICI 182,780 (CAS #: 72795-01-8; purity 99 %; catalog no. 1047) antagonist control, methanol control, or bottled water extract. Extracts were resuspended in methanol, diluted in bioassay media, and screened at 5 and 10 times the final concentration of the original water sample. Each sample was dosed in quadruplicate and analyzed across at least 3 replicate 96-well plates (i.e., unique cell passage). Luminescence (relative light units; RLU) was measured using ClarioStar luminometer (BMG LabTech, Cary, NC). Data and statistical analysis were performed using GraphPad Prism 8.0 (GraphPad Software, La Jolla, California) and SAS statistical software (Cary, NC USA), as previously described (Medlock Kakaley et al. 2021). All results are in Tables S3–S5 and in Romanok et al. (2022).
2.3. Quality assurance
Quantitative (≥limit of quantitation, ≥LOQ) and semi-quantitative (between LOQ and long-term method detection limit, MDL (Childress et al. 1999; U.S. Environmental Protection Agency 2020b)) results were treated as detections (Childress et al. 1999; Foreman et al. 2021; Mueller et al. 2015). Chemical quality-assurance/quality-control included 3 nominal field blanks (organics, inorganics) as well as laboratory blanks (organics, inorganics), spikes (organics), and stable–isotope surrogates (organics) prepared at respective analytical laboratories. The median organic surrogate recovery (Table S4c) was 98.5 % (interquartile range: 88.5–108 %). Despite infrequent detections and very low detected concentrations in inorganic blanks, maximum blank concentrations for bromide, sulfate, and zinc were nevertheless within the range observed in some BW samples (Table S3c); corresponding results were censored at the analyte-specific maximum blank concentration, as footnoted (Table S3a). Only chlorodifluoromethane (HCFC-22; 0.02 μg L−1), 1,1-difluoroethane (0.01 μg L−1), ethyl acetate (0.09 μg L−1), and n-pentanal (0.011 μg L−1) were detected in blanks (once each) at concentrations in the range observed in BW samples (Table S4b); corresponding results were censored at twice the analyte-specific maximum blank concentration and, consequently, HCFC-22 was removed from the interpretive dataset, as footnoted (Table S4a). No growth was detected for any microbial quality-assurance/quality-control sterile laboratory blank, as footnoted (Tables S5).
2.4. Statistics
Differences (centroids and dispersions) between BW-sample groups were assessed by nonparametric One–way PERMANOVA (n = 9999 permutations) on Euclidean distance (Paleontological Statistics, PAST, vers. 4.03) (Hammer et al. 2001). Relations between detected BW contaminants were assessed by Spearman rank-order (rho (ρ)) correlation and permuted (n = 9999 permutations) probabilities (PAST, vers. 4.03) (Hammer et al. 2001).
2.5. Risk assessments
A screening-level assessment (Goumenou and Tsatsakis 2019; U.S. Environmental Protection Agency 2011) of potential cumulative biological activity of mixed-organic contaminants in each BW sample was conducted as described (Blackwell et al. 2017; Bradley et al. 2018; Bradley et al., 2019). The toxEval version 1.2.0 package (De Cicco et al. 2018) of the open source statistical software R (R Development Core Team 2019) was used to sum (non-interactive concentration addition model (e.g., Altenburger et al. 2018; Cedergreen et al. 2008; Stalter et al. 2020) individual EAR (ratio of the detected concentration to the activity concentration at cutoff (ACC) from the Toxicity ForeCaster (U.S. Environmental Protection Agency 2020a; U.S. Environmental Protection Agency National Center for Computational Toxicology 2020) high-throughput screening data (U.S. Environmental Protection Agency National Center for Computational Toxicology 2020)) to estimate sample-specific cumulative EAR (ΣEAR) (Blackwell et al. 2017; Bradley et al. 2018; Bradley et al. 2020). ACC estimates the point of departure concentration at which a defined threshold of response (cutoff) is achieved for a given biological activity and is less prone to violations of relative potency assumptions (for discussion see, Blackwell et al. 2017). ACC data in the toxEval v1.2.0 employed in the present study were from the August 2020 invitroDBv3.2 release of the ToxCast database (U.S. Environmental Protection Agency 2020a; U.S. Environmental Protection Agency National Center for Computational Toxicology 2020). Non-specific-endpoint, baseline, and unreliable response-curve assays were excluded (Blackwell et al. 2017; Bradley et al. 2021a; Bradley et al. 2021b). ΣEAR results and exclusions are summarized in Tables S6a–6b.
An analogous human-health-benchmark HI assessment (Goumenou and Tsatsakis 2019; U.S. Environmental Protection Agency 2011, 2012) of the combined inorganic and organic contaminant risk also was conducted using toxEval v1.2.0 (De Cicco et al. 2018) to sum the toxicity quotient (TQ, ratio of detected concentration to corresponding health–based benchmark) of individual detections to estimate sample-specific cumulative TQ (ΣTQ) (Corsi et al. 2019). A precautionary screening–level approach was employed based on the most protective human–health benchmark (i.e., lowest benchmark concentration) among maximum contaminant level (MCL) goal (MCLG) (U.S. Environmental Protection Agency 2017, 2021e), World Health Organization (WHO) Guideline Values (GV) and provisional GV (pGV) (World Health Organization (WHO) 2011), USGS Health-Based Screening Level (HBSL; (Norman et al. 2018)), and state drinking-water MCL or drinking-water health advisories (DWHA). For the ΣTQ assessment, MCLG values of zero (i.e., no identified safe-exposure level for sensitive sub-populations, including infants, children, the elderly, and those with compromised immune systems and chronic diseases (U.S. Environmental Protection Agency 2021d, e)) were set to the respective method reporting limit, except for Pb, which was set to 1 μg L−1 as suggested by the American Academy of Pediatrics (Lanphear et al. 2016). ΣTQ results and respective health–based benchmarks are summarized in Tables 7a–7b. Corsi et al. (2019) reported approximate contaminant-specific equivalency of the widely employed TQ = 0.1 screening-level threshold of concern and EAR = 0.001.
3. Results and discussion
Consistent with an increasingly anthropized water cycle and with the results of previous POU–TW studies by this group, regulated and unregulated chemical (inorganic, organic) and microbial analytes were routinely detected in BW samples (Tables S3–S5; Figures 1–4, S1), with 2 or more detections of potential human–health concern often observed per sample. Approximately 91 % (48) of the 53 inorganic analytes and 10 % (45) of the 465 unique organic–indicator analytes were detected at least once in BW. Bacteria were broadly detected in BW by direct counts (83 % of samples) and by growth on non-selective heterotrophic plate media (70 %), with detection of growth on at least one putative pathogen selective media in 24 (80 %) of the tested BW samples.
Fig. 1.
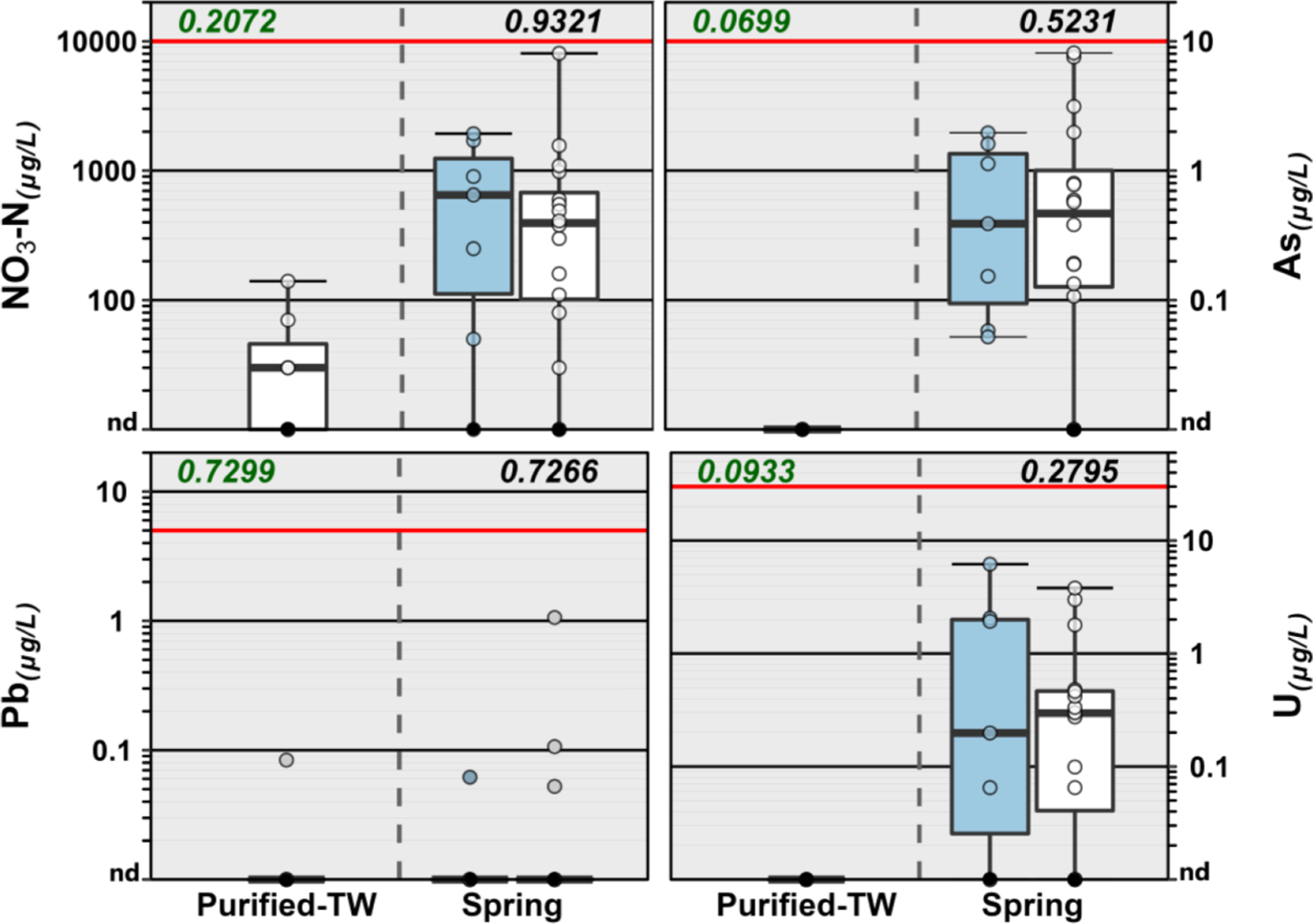
Group comparison of concentrations of select inorganics detected in purified-TW (domestic) and spring (domestic, white; imported, blue) sourced bottled water samples during 2020. Solid red lines indicate enforceable FDA SOQ levels. EPA MCLG for As, U, and Pb are zero. For NO3-N, SOQ and MCLG are the same. Boxes, centerlines, and whiskers indicate interquartile range, median, and 5th and 95th percentiles, respectively. Numbers in green font (top left of plots) indicate the permuted probability that the centroids and dispersions are the same (PERMANOVA; 9999 permutations) across all (purified-TW and spring sourced) BW groups; numbers above spring-sourced BW boxplot pairs (top right of plots) indicate the permuted probability that the centroids and dispersions are the same for spring-sourced BW groups. “nd” indicates not detected.
Fig. 4.
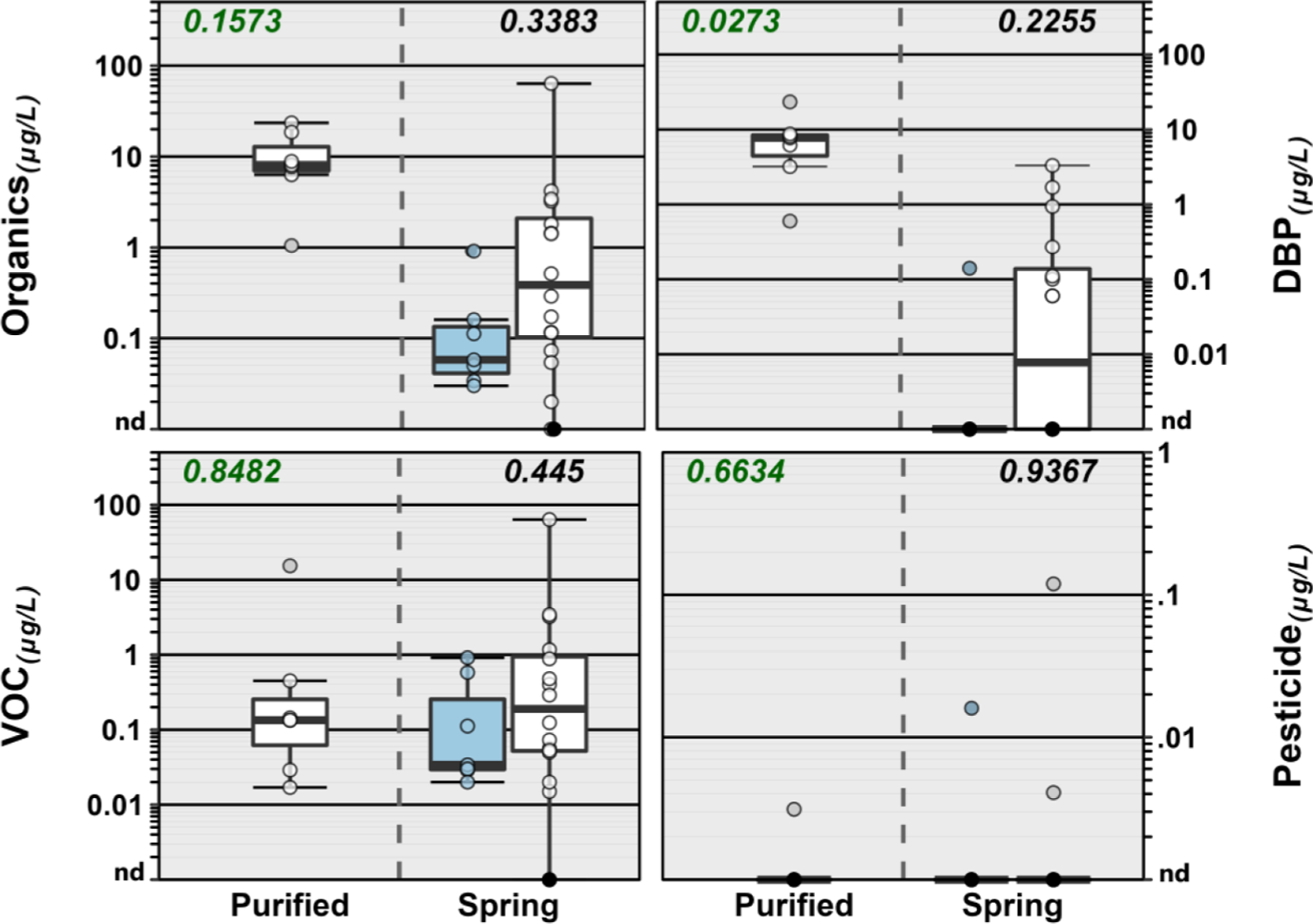
Group comparisons of cumulative concentration of all organics (upper left plot) and select organic classes detected in spring (domestic, white; imported, blue) and purified-TW (domestic) sourced bottled water samples during 2020. Boxes, centerlines, and whiskers indicate interquartile range, median, and 5th and 95th percentiles, respectively. Numbers in green font (top left of plots) indicate the permuted probability that the centroids and dispersions are the same (PERMANOVA; 9999 permutations) across all (purified-TW and spring sourced) BW groups; numbers above spring-sourced BW boxplot pairs (top right of plots) indicate the permuted probability that the centroids and dispersions are the same for spring-sourced BW groups. “nd” indicates not detected.
Organ/organism–level human–health effects are screened herein based on MCLG and other human-health drinking-water advisories that define a margin-of-exposure concentration below which there is no known risk to the health of presumptive “most vulnerable” (e.g., infants, children, pregnant women, elderly, immune-compromised) sub-populations (U.S. Environmental Protection Agency 2021d). Consistent with previous publications by this group, FDA SOQ (i.e., “shall not contain in excess of”) levels (U.S. Food & Drug Administration 2021) are presented to provide regulatory context; however, the EPA MCL values, on which FDA SOQ are generally based, take available treatment technologies and cost into consideration and, consequently, often are greater than corresponding human-health-only EPA MCLG values (U.S. Environmental Protection Agency 2021e).
3.1. BW exposure-benchmark comparisons – inorganics
No exceedances of FDA SOQ levels were observed for any inorganic analytes (Fig. 1, Table S3a). Few exceedances of human–health advisories for inorganics were observed in BW samples, with the notable exception of arsenic (As), uranium (U), and lead (Pb), which were broadly-detected here and widely reported at < MCL (less than the treatment technique action level for Pb) concentrations in previous BW studies in the US (e.g., Ikem et al. 2002; Saleh et al. 2008) and globally (e.g., Felipe-Sotelo et al. 2015; Krachler and Shotyk 2009) and which have no known safe level of exposure for vulnerable sub-populations (i. e., MCLG zero) (U.S. Environmental Protection Agency 2018).
Arsenic was not detected in any purified-TW BW (domestic) but was frequently detected (≥0.1 μg L−1) in domestic and imported spring-sourced BW (87 %), at concentrations up to greater than 7 μg L−1 in two domestic samples. Drinking-water As exposure is associated with various cancers (Mohammed Abdul et al. 2015; Smith and Steinmaus 2009), organ–system toxicity (Mohammed Abdul et al. 2015), cardiovascular disease (Navas-Acien et al. 2005; Pichler et al. 2019), diabetes (Navas-Acien et al. 2005; Pichler et al. 2019), adverse pregnancy outcomes (Shih et al. 2017), and mortality (Argos et al. 2010; Shih et al. 2017). Growing concerns for adverse health effects of drinking-water As concentrations less than the 10 μg L−1 EPA MCL (García-Esquinas et al. 2013; Mohammed Abdul et al. 2015; Navas-Acien et al. 2005; Navas-Acien et al. 2008) have prompted more strict public-supply MCL (e.g., 5 μg L−1 in New Hampshire and New Jersey) in some US states (New Hampshire Department of Environmental Services 2021; State of New Jersey 2021).
Likewise, U was frequently (74 %) detected (≥0.1 μg L−1) in domestic and imported spring–sourced BW, at concentrations up to 6.2 μg L−1 (imported) but was not detected in any purified–TW BW. Drinking-water U is associated with human nephrotoxicity (Magdo et al. 2007; Seldén et al. 2009) and osteotoxicity (Kurttio et al. 2005), DNA-repair inhibition in human embryonic kidney 293 (HEK293) cells (Cooper et al. 2016), and estrogen-receptor effects in mice (Raymond-Whish et al. 2007). Notably, As and U co-occurred in about 70 % (16) of spring–sourced BW samples in this study.
Pb was detected (≥0.1 μg L−1) in 5 of the 30 BW brands (17 %) at concentrations up to 1.1 μg L−1 and with comparable frequency in purified–TW and spring–sourced BW (14 % and 17 %, respectively). Public–health concerns for elevated drinking-water Pb–exposures are focused primarily on neurocognitive impairment in infants and children (Lanphear et al. 2016; Triantafyllidou and Edwards 2012), with the American Academy of Pediatrics recommending that drinking-water Pb not exceed 1 μg L−1 (Lanphear et al. 2016). Drinking-water Pb is attributed primarily to premise–plumbing and distribution–system infrastructures (Triantafyllidou and Edwards 2012) that predate the 1986 SDWA Amendments (U.S. Environmental Protection Agency 2021c).
Nitrate was routinely detected in BW samples in this study at concentrations generally consistent with previous BW comparison studies in the US (e.g., Ikem et al. 2002; Saleh et al. 2008) and globally (e.g., Felipe-Sotelo et al. 2015; Krachler and Shotyk 2009). Concentrations of NO3-N greater than 1 mg L−1 (including one at 8.1 mg L−1) were observed in 22 % (5/23) of spring–sourced BW samples in this study but not in any purified–TW BW. While the 10 mg L−1 MCLG was established to protect bottle-fed infants (<6 months) against methemoglobinemia (U.S. Environmental Protection Agency 2018, 2021e), drinking-water exposures to < MCLG NO3–N concentrations recently have been associated with several adverse outcomes (Ward et al. 2005; Ward et al. 2018), including cancers (Jones et al. 2016; Jones et al. 2017; Quist et al. 2018), thyroid disease (Aschebrook-Kilfoy et al. 2012), and neural tube defects (Brender et al. 2013).
Fluoride concentrations in all BW samples were below the 0.7 mg L−1 US Public Health Service drinking-water optimum to prevent childhood dental caries (2015), in line with previous concerns for the dental health of children, for whom BW is the primary drinking–water source (Cochrane et al. 2006; Horowitz et al. 2015; Mills et al. 2010). F concentrations were < 0.6 mg L−1 in all but one BW sample and < 0.3 mg L−1 in 28 (93 %) samples. F supplementation from 3 to 16 years of age is recommended for children with drinking-water F < 0.6 mg L−1, beginning at 6 months if F is < 0.3 mg L–1 (American Academy of Pediatrics: Committee on Nutrition 1995; Kohn et al. 2001).
3.2. BW exposure-benchmark comparisons - organics
Twenty-one (47 %) of the 45 organic analytes detected in this study were detected in 2 or fewer samples, with 14 (31 %) detected only once (Fig. 2, Table S4a). All but one BW sample (97 %) had at least one organic analyte detection, with more than one analyte detected in 87 % (26/45) of samples (Fig. 3 and Fig. 4). On average (median), 5 organics were detected per sample (interquartile range [IQR]: 2 – 6; range: 0 – 22), consistent with Hypothesis I, an anthropized water cycle, and previous private-/public-supply TW results by this research group (Bradley et al. 2018; Bradley et al. 2020; Bradley et al. 2021a; Bradley et al. 2021b; Bradley et al. 2022). In general, the most frequently detected organic analytes were DBP residuals of chlorine disinfection (e. g., trichloromethane, bromodichloromethane, acetonitrile, tribromo-methane), detected primarily in purified-TW BW but also in some spring-sourced BW (median: 4, IQR: 3 – 4 versus spring-sourced median: 0, IQR: 0 – 1), and a range of volatile organic chemical(s) (VOC) in both purified-TW (median: 2, IQR: 1 – 3) and spring–sourced (median: 2, IQR: 1 – 5) BW samples. Detection of trihalomethane DBP in purportedly untreated (i.e., no chlorine disinfection) spring-sourced BW samples has been reported previously (Stanhope et al. 2020). The total trihalomethane DBP concentration in one purified–TW BW sample was more than double the International Bottled Water Association’s code of practice limit of 10 μg L−1 (International Bottled Water Association 2020). In contrast to several previous studies (e.g., Akhbarizadeh et al. 2020; Chow et al. 2021; Gellrich et al. 2013; Luo et al. 2018; Wang et al. 2021), no PFAS, pharmaceutical, or phthalate contaminants were detected in this BW study. Likewise, pesticides were detected infrequently (4 samples), with the notable exception of one domestic, spring-sourced BW with 5 pesticide detections (cumulative concentration 0.119 μg L−1).
Fig. 2.
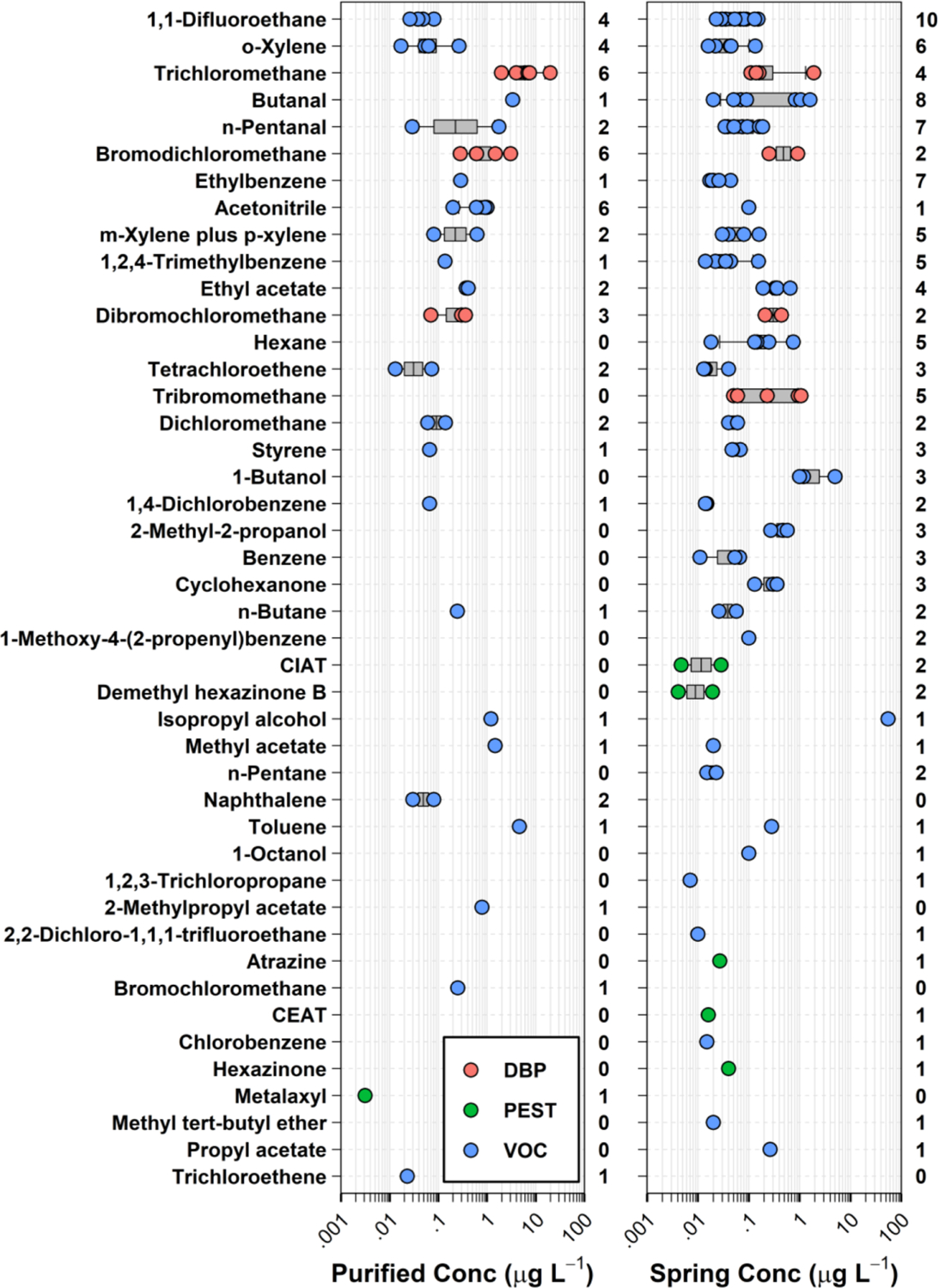
Detected concentrations (circles, μg L−1) and number of bottled water samples (right axes) for 45 organic analytes (left axis, in order of decreasing total detections) detected in purified-TW (left plot) and spring (right plot) sourced bottled water samples during 2020. Circles (●) are data for individual samples. Boxes, centerlines, and whiskers indicate interquartile range, median, and 5th and 95th percentiles, respectively. DBP, PEST, and VOC indicate disinfection byproducts, pesticides, and volatile organic chemicals, respectively. VOC generally associated with disinfection processes when present in drinking water are identified as DBP. CEAT and CIAT are deisopropylatrazine and deethylatrazine, respectively.
Fig. 3.
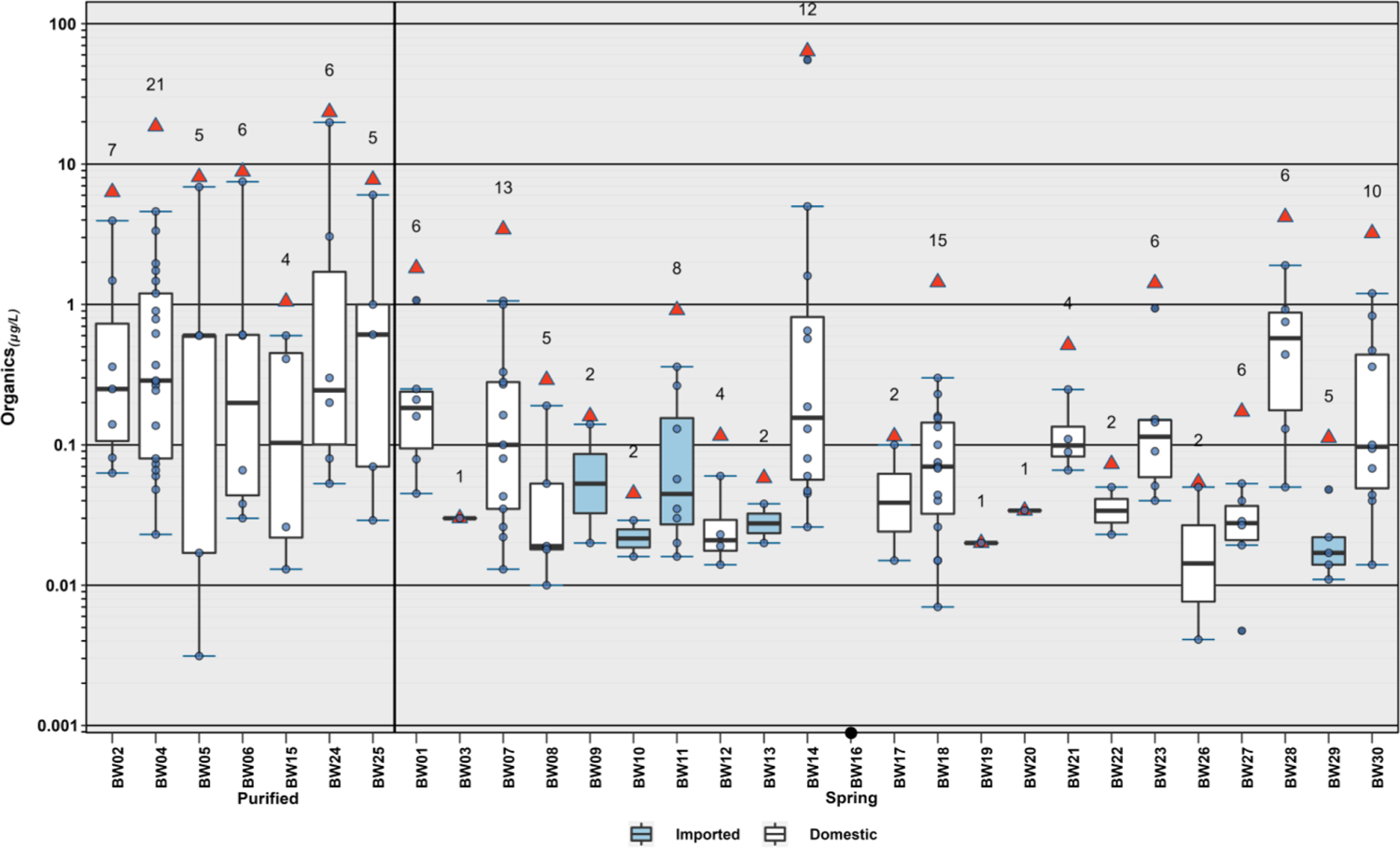
Individual (circles, ●) and cumulative (sum of all detected; red triangles, △) concentrations of 45 organic analytes detected in spring (domestic, white; imported, blue) and purified-TW (domestic, white) sourced bottled water samples during 2020. Boxes, centerlines, and whiskers indicate interquartile range, median, and 5th and 95th percentiles, respectively. Numbers above each boxplot indicate total detected organic analytes. Circle on x-axis (BW16) indicates no organic analytes detected.
In line with Hypothesis II, no exceedances of FDA SOQ levels were observed for organic analytes in this study. Fifteen of the 45 organics detected in this study (33 %) have EPA promulgated MCLG. Among these, 3 DBP (bromodichloromethane [8 samples], tribromomethane [5], dichloromethane [4]) and 3 VOC (tetrachloroethene [5], benzene [3], trichloroethene [1]) have no known safe level of exposure for vulnerable sub-populations (i.e., MCLG of zero) (U.S. Environmental Protection Agency 2018), representing de facto exceedances (Hypothesis III). Simultaneous exposures to multiple organic contaminants in these samples expand the concern-space for potential biological effects of POU drinking water exposures to include BW, emphasizing the need for improved understanding of the adverse human-health implications, if any, of long-term exposures to low–level organic-contaminant mixtures across all 3 drinking-water distribution pipelines (private–, public–, and bottled–supply).
3.3. BW exposure-benchmark comparisons - microbial
Viable bacteria were detected by heterotrophic plate count (HPC) or by microscopic direct counts in 97 % (29/30) of BW samples (6/7 purified-TW, 23/23 spring–sourced) and at concentrations greater than 100 HPC CFU 100 mL−1 in 17 % of samples, all spring-sourced (Table S5). HPC bacteria occur naturally in the environment, are commonly detected in drinking water, and are not intrinsic health concerns but are useful indicators of source-water quality, system maintenance, disinfection efficacy, and post-treatment regrowth in the distribution “pipeline” prior to consumption (U.S. Environmental Protection Agency 2021e). To optimize detection of viable heterotrophs, BW samples were assessed with two growth media (SimPlate, R2A) and incubation durations (2 and 4 days). In general, detections of heterotrophs by HPC and of bacteria and virus-like particles by direct microscopic counts were more common in spring–sourced BW than in purified-TW BW; the latter were all derived from chlorine-disinfected TW (as indicated by presence of chlorine DBP) and treated by reverse-osmosis advanced filtration (according to label). Reduction and avoidance of chlorine-disinfection DBP and associated tastes/odors are common considerations, respectively, for advanced filtration (e.g., reverse osmosis) of purified-TW BW and for use of non-chlorine, advanced–oxidation (ozonation, ozonation/UV radiation) for spring–sourced BW disinfection, when employed. While two of the highest HPC results were observed in spring-sourced BW with no listed filtration or treatment (BW03, BW29), comparable high results for ozone–/UV–disinfected BW illustrate the trade-off of reduced DBP/taste concerns but increased biological regrowth concerns in the absence of residual disinfectant. Growth on putative pathogen selective media was observed sporadically across all BW samples, albeit at near detection-limit levels.
3.4. BW in vitro bioactivities
Given the potential low estrogenic activity in the sample extracts, a tiered screening approach was applied to sample analysis as previously described (Medlock Kakaley et al. 2021). No BW sample extract produced estrogenic activity significantly greater than control treated cells (p < 0.01) and, therefore, none exceeded the bioassay minimum detectable concentration (MDC; 0.057 ng/L) for estrogenic activity. Estrogenic activity, generally below estimated trigger values for adverse effects (Neale and Escher 2019), has been reported previously in treated TW in the US (Conley et al. 2017b) and globally (Brand et al. 2013; Maggioni et al. 2013; Van Zijl et al. 2017) and in BW (Aneck-Hahn et al. 2018; Real et al. 2015; Wagner and Oehlmann 2009).
3.5. BW aggregated screening assessments
Potential human-health effects of BW contaminant–mixture exposures were screened using cumulative bioactivity-weighted approaches based on detected analytes. The ΣEAR and ΣTQ approaches employed herein and in the previous TW studies 1) are constrained intrinsically by the analytical scope, which, although extensive (in this case 465 unique organics and 53 inorganics), remains orders-of-magnitude below estimates of anthropogenic chemicals in commercial production (Wang et al. 2020) and, by extension, potentially present in ambient drinking-water source waters (Bradley et al., 2017; de Jesus Gaffney et al. 2015; DeSimone et al. 2015; Toccalino et al. 2012), 2) are limited by available weighting–factors (ToxCast ACC and human health benchmarks, respectively), and 3) estimate mixture effects assuming approximate concentration addition (e.g., Cedergreen et al. 2008; Ermler et al. 2011; Stalter et al. 2020). The ΣEAR approach (Blackwell et al. 2017; Bradley et al. 2021a) employs ToxCast high-throughput exposure-effects data to predict potential cumulative bioactivity at the molecular scale (Filer et al. 2017; Richard et al. 2016); however, ToxCast has no coverage of inorganic contaminants and not all predicted organic-contaminant molecular responses are necessarily adverse at organ/organism scales (Schroeder et al. 2016). We aggregated contaminant bioactivity ratios across all ToxCast endpoints without restriction to recognized modes of action to provide a precautionary lower-bound estimate of in vivo adverse-effect levels (Paul Friedman et al. 2020), but this approach may not accurately reflect apical effects (Blackwell et al. 2017; Schroeder et al. 2016). In contrast, the employed ΣTQ approach targets apical human-health effects, includes inorganic exposures, but is notably constrained to recognized (i.e., benchmarked) health concerns. Importantly, the EAR approach is based on measured endpoint-specific activity cutoff concentrations, whereas the human-health benchmarks used in the TQ approach include a margin of safety (margin of exposure).
Twenty-six of the 45 organic contaminants detected in BW samples had exact Chemical Abstract Services (CAS) number matches in the ToxCast invitroDBv3.2 database (Figure S2, Table S6b). The highest individual EAR values (1.35–6.74) and the only EAR and ΣEAR exceeding the level expected to modulate molecular targets in vitro (i.e., solid red ΣEAR = 1 line, Fig. 5) in this study were for three spring-sourced BW samples containing μg L−1 concentrations of the VOC, 1–butanol. Acknowledging the incomplete (58 %) ToxCast coverage of the detected organic analytes, the potential 2–3 orders–of–magnitude analytical underestimation of the presumptive exposure space (465 unique analytes compared to estimated 350,000 commercial organic compounds and presumptive greater number (Dobson 2004) of corresponding degradates and metabolites in the environment), the recognized vulnerability of specific populations (Blake and Fenton 2020), and the reported approximate contaminant-specific equivalency to the widely employed TQ = 0.1 screening-level threshold for low risk (Corsi et al. 2019), a precautionary ΣEAR = 0.001 screening–level was employed, as described. Exceedances of ΣEAR = 0.001 in more than half (17/30) of the BW samples were attributable primarily to DBP in purified-TW BW and to a variety of VOC, including trihalomethanes, in spring-sourced BW and were most consistently associated with DNA-binding endpoints (Table 6c); based on these results, further investigation of the cumulative molecular activity of low-level BW chemical exposures is warranted.
Fig. 5.
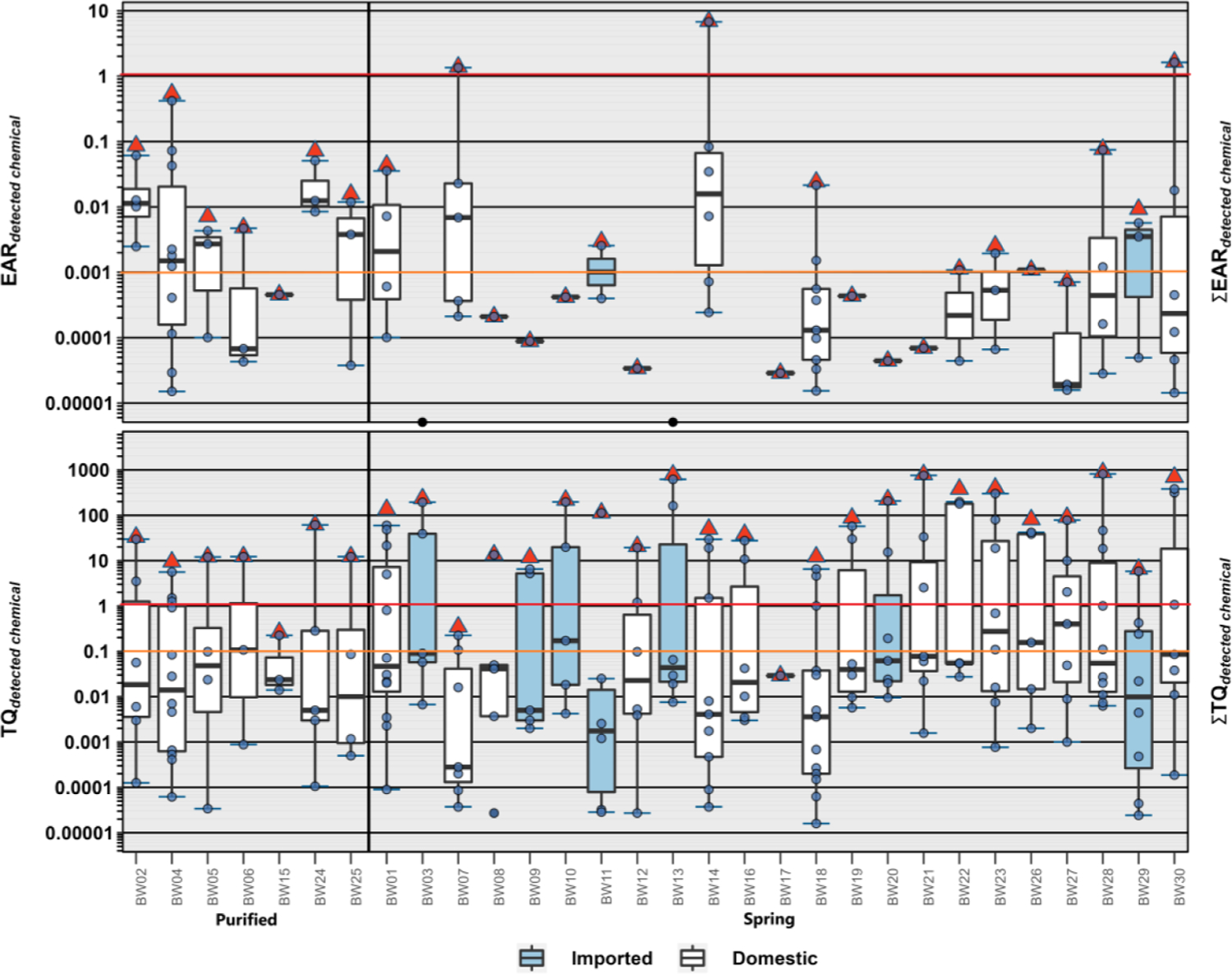
Top. Individual EAR values (circles) and cumulative EAR (ΣEAR, sum of all detected; red triangles, △) across all assays for 24 organic analytes listed in ToxCast and detected in spring (domestic, white; imported, blue) and purified-TW (domestic) sourced bottled water samples during 2020. Solid and dashed red lines indicate concentrations shown to modulate effects in vitro and effects-screening-level thresholds (EAR = 0.001), respectively. Circles on x-axis (BW03 and BW13) indicate EAR < 0.00001. Bottom. Human health benchmark-based individual TQ values (circles) and cumulative TQ (ΣTQ, sum of all detected; red triangles, △) for inorganic and organic analytes listed in Table S7a and detected in spring (domestic, white; imported, blue) and purified-TW (domestic) sourced bottled water samples during 2020. Solid and dashed red lines indicate benchmark equivalent concentrations and effects-screening-level threshold of concern (TQ = 0.1), respectively. Boxes, centerlines, and whiskers indicate interquartile range, median, and 5th and 95th percentiles, respectively, for both plots.
All but one of the BW samples in this study exceeded the ΣTQ = 0.1 screening threshold of potential concern, with most (27/30) exceeding ΣTQ = 1 (Figures 5, S3; Table S7b). These ΣTQ results indicate high probabilities of cumulative risk in the tested BW samples, when considering both organic and inorganic contaminant exposures. Exceedances of ΣTQ = 1 were driven primarily by DBP in purified-TW BW samples, all of which were labeled as RO treated; incomplete or poor RO rejection of low molecular weight organics including trihalomethane DBP is well-documented (Breitner et al. 2019; Marron et al. 2019). Exceedances of ΣTQ = 1 in spring–sourced BW samples also were primarily attributable to trihalomethane compounds, with tribromomethane and bromodichloromethane, compounds with no known safe level of exposure (MCLG = 0), alone exceeding the threshold in 5 (22 %) and 2 (8 %) spring-sourced BW samples, respectively. Other notable ΣTQ results included frequent, often co-occurring, detections of As, Pb, and U, as noted above. These results indicate that simultaneous exposures to multiple drinking-water contaminants of potential human-health concern are common in BW, emphasizing the need for improved understanding of the adverse human-health implications, if any, of long-term exposures to low–level inorganic-/organic-contaminant mixtures across all three drinking-water distribution pipelines (public TW, private TW, BW).
3.6. Study limitations and future research
For this initial reconnaissance of the potential for human exposures to an expanded range of inorganic, organic, and microbial contaminants in BW, 30 BW brands were selected to broadly represent the range of source locations, source waters, pre-distribution treatments, and packaging materials of commercially available BW across the US. However, BW is the largest commercial beverage category by volume in the US and the 30 brands assessed herein are a small fraction of those available in the US and globally (Rodwan 2021); further latitudinal (more brands) and longitudinal (temporal variability) assessment is required to fully inform the range of BW exposures in the US and globally. Likewise, as noted above, the target analytical scope of this and the previous POU–TW studies (Bradley et al. 2018; Bradley et al. 2020; Bradley et al. 2021a; Bradley et al. 2021b; Bradley et al. 2022), while extensive and environmentally relevant, is only a fractional indicator of anthropogenic chemicals in commercial production and potentially present in ambient drinking-water source waters; accordingly, the exposure and associated risk results may be reasonably interpreted as potential orders-of-magnitude underestimates. Other inherent limitations of the EAR and TQ risk assessment approaches are discussed in the previous section. Additionally, the EAR risk assessment approach herein and in the previous POU–TW studies (Bradley et al. 2018; Bradley et al. 2020; Bradley et al. 2021a; Bradley et al. 2021b; Bradley et al. 2022) employs measured BW concentrations as a direct estimate of human exposure. Alternatively, the ToxCast molecular–endpoint ACC data may be viewed as more aligned with internal doses and the EAR risk assessment conducted using internal doses estimated by, for example, high-throughput toxicokinetic modeling using the httk R package (Pearce et al. 2017), as described (Paul Friedman et al. 2020). Lastly, the ToxCast datasets and to a more limited extent the benchmarks employed in the ΣEAR and ΣTQ risk assessments, respectively, have been routinely updated; accordingly, in contrast to the exposure results, direct comparison of estimated risks between this and the previous POU–TW studies requires harmonization of the ACC and benchmark data and was beyond the scope of the current study. Important next steps include harmonization of toxicity-weighting data and direct comparison of the ΣEAR and ΣTQ risk assessments based on POU–DW exposure data, comparative assessment of EAR risk based on the internal dose estimation approach of Paul Friedman et al. 2020, continued target assessment of broad inorganic, organic, microbial exposures and associated risks across all three POU-DW distribution pipelines, and incorporation of non-targeted chemical and effects-based assay platforms to more broadly characterize human POU-DW exposures and risks.
4. Conclusions
In the US and globally, drinking water is delivered to consumers via three distribution systems (public TW, private TW, BW), each having distinct logistical, infrastructure, regulatory, and commercial profiles, but all similarly challenged by anthropogenic water-quality concerns. Attributed largely to commercial promotion as a safer alternative to public and private TW (Hawkins 2017; Olson et al. 1999), BW consumption has increased dramatically in the US (Rodwan 2021) amidst heightened anxieties about environmental contaminant exposures and health risks (Pape and Seo 2015; Zivin et al. 2011), despite a paucity of directly comparable, realistically-broad contaminant exposure data for BW. This data gap impedes individual-consumer risk–management decision making.
In this study, 48 inorganics and 45 organics, including some with documented human-health concerns, were detected in sampled BW. While no FDA SOQ levels were exceeded in any BW samples, several inorganic and organic contaminants with MCLG of zero (no known safe level of exposure to vulnerable sub-populations) were detected, in some cases at near–MCL concentrations. Among these, As, Pb, and U were detected in 67 %, 17 %, and 57 % of BW samples, respectively, and almost exclusively in spring-sourced BW samples, which were not treated by the advanced filtration (e.g., reverse osmosis) methods applied to all the purified–TW sourced BW samples assessed herein. Organic MCLG exceedances included frequent detections of DBP in TW–sourced BW and infrequent detections of VOC in purified–tapwater and spring–sourced BW. Precautionary health–based HI screening levels were exceeded frequently and attributed primarily to chlorine–disinfection DBP in purified-tapwater sourced BW and to co-occurring inorganic (e.g., As, U) and organic (e.g., brominated trihalomethanes) contaminants in spring-sourced BW. While extensive (465 unique organics, 53 inorganics), the organic analytes assessed in this study are an orders–of–magnitude underestimate of the breadth of anthropogenic chemicals in commercial production (Wang et al. 2020) and, thus, presumptive fractional indicators of potential BW exposures and risk. The results of this one-time reconnaissance of a limited number of BW brands (30) indicate that simultaneous exposures to multiple drinking-water contaminants of potential human-health concern are common in BW and illustrate the need for further directly–comparable, realistically-broad contaminant exposure assessments across a broader range of BW brands and over time.
Importantly, comparison of these BW exposure results with those of the previous POU–TW studies documents the shared challenge to all three POU-DW distribution pipelines posed by the increasingly anthropized water cycle. To illustrate, Fig. 6 displays sample results for select major ion (NO3-N), trace metal (As), and organic (VOC) contaminants detected in this BW study and in the previous POU–TW studies (Bradley et al. 2018; Bradley et al. 2020; Bradley et al. 2021a; Bradley et al. 2021b; Bradley et al. 2022). Considerable variability in POU-DW contaminant exposures exists for private, public, and bottled DW supplies, with generally comparable ranges in contaminant concentrations observed to date across all three distribution pipelines. The results to date do not support market-driven perceptions of BW as systematically higher purity than public TW and emphasize the need for improved source-water protection, monitoring and characterization, and treatment options across all three pipelines.
Fig. 6.
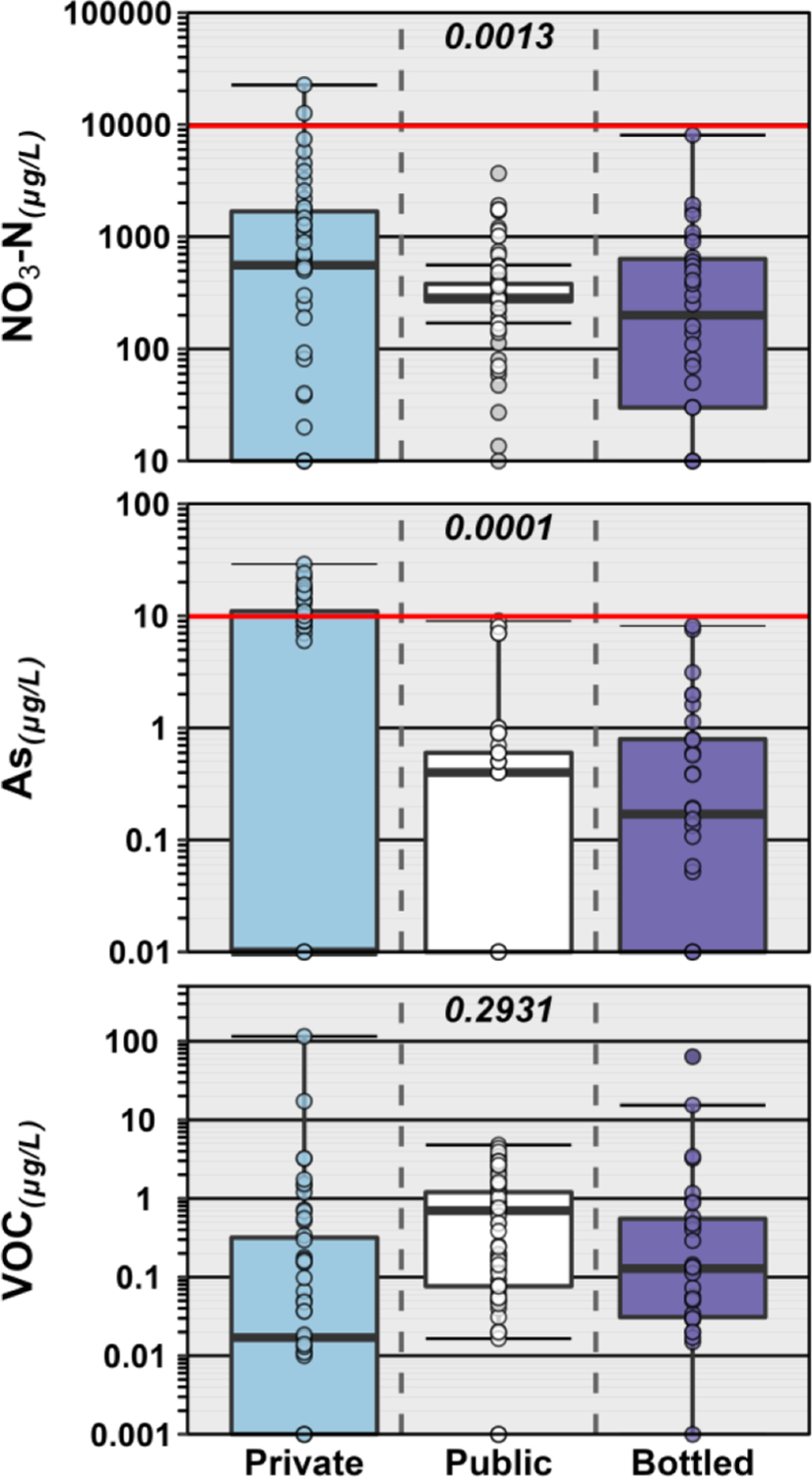
Group comparison of concentrations of NO3-N (top), As (middle), and VOC (bottom) detected in private TW (blue) and public TW (white) in previously published studies and in BW (purple) herein. Solid red lines indicate enforceable FDA SOQ levels. For NO3-N, SOQ and MCLG are the same. MCLG for As is zero. Boxes, centerlines, and whiskers indicate interquartile range, median, and 5th and 95th percentiles, respectively. Numbers (top center of plots) indicate the permuted probability that the centroids and dispersions are the same (PERMANOVA; 9999 permutations) across all groups.
Improved understandings of point-of-use drinking-water contaminant exposures based on more environmentally realistic and directly comparable POU-exposure characterizations, like this BW study and previous TW studies by this group (Bradley et al. 2018; Bradley et al. 2020; Bradley et al. 2021a; Bradley et al. 2021b; Bradley et al. 2022), are essential to public health, because drinking-water is a biological necessity and, consequently, a high-vulnerability vector for human contaminant exposures (Dai et al. 2017). The results illustrate the importance of continued systematic, quantitative assessments of realistically-broad contaminant exposures and associated bioactivities in POU drinking water from all three distribution pipelines (private TW, public TW, and BW) to support models of drinking-water contaminant exposures and related risks at the point of use.
Supplementary Material
Acknowledgements
This research was conducted and funded by the USGS Ecosystems Mission Area, Environmental Health Program. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. The findings and conclusions in this article do not necessarily represent the views or policies of the US Environmental Protection Agency, Food and Drug Administration, or National Institute of Environmental Health Sciences. This report contains CAS Registry Numbers®, which is a registered trademark of the American Chemical Society. CAS recommends the verification of the CASRNs through CAS Client ServicesSM.
Footnotes
CRediT authorship contribution statement
Paul M. Bradley: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing. Kristin M. Romanok: Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – review & editing. Kelly L. Smalling: Formal analysis, Investigation, Methodology, Project administration, Writing – review & editing. Michael J. Focazio: Writing – review & editing. Nicola Evans: Investigation, Writing – review & editing. Suzanne C. Fitzpatrick: Writing – review & editing. Carrie E. Givens: Investigation, Writing – review & editing. Stephanie E. Gordon: Visualization, Writing – review & editing. James L. Gray: Investigation, Writing – review & editing. Emily M. Green: Investigation, Writing – review & editing. Dale W. Griffin: Investigation. Michelle L. Hladik: Investigation, Writing – review & editing. Leslie K. Kanagy: Investigation. John T. Lisle: Investigation, Writing – review & editing. Keith A. Loftin: Investigation, Writing – review & editing. R. Blaine McCleskey: Formal analysis, Investigation, Writing – review & editing. Elizabeth K. Medlock–Kakaley: Investigation, Writing – review & editing. Ana Navas-Acien: Writing – review & editing. David A. Roth: Investigation, Writing – review & editing. Paul South: Writing – review & editing. Christopher P. Weis: Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2022.107701.
Data availability
All data are available in the supporting information file and in the cited Romanok et al. 2022 USGS Data Release.
References
- Akhbarizadeh R, Dobaradaran S, Schmidt TC, Nabipour I, Spitz J, 2020. Worldwide bottled water occurrence of emerging contaminants: A review of the recent scientific literature. J. Hazard. Mater 392, 122271 10.1016/j.jhazmat.2020.122271. [DOI] [PubMed] [Google Scholar]
- Allaire M, Wu H, Lall U, 2018. National trends in drinking water quality violations. PNAS 115 (9), 2078–2083. 10.1073/pnas.1719805115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altenburger R, Scholze M, Busch W, Escher BI, Jakobs G, Krauss M, et al. , 2018. Mixture effects in samples of multiple contaminants–An inter-laboratory study with manifold bioassays. Environ. Int 114, 95–106. 10.1016/j.envint.2018.02.013. [DOI] [PubMed] [Google Scholar]
- American Academy of Pediatrics: Committee on Nutrition. 1995. Fluoride supplementation for children: Interim policy recommendations. Pediatrics. 95 (5), 777–777. 10.1542/peds.95.5.777. [DOI] [PubMed] [Google Scholar]
- American Public Health Association 2018a. 9213 Recreational Waters (2017). In: Standard Methods For the Examination of Water and Wastewater, 8, https://www.standardmethods.org/doi/abs/10.2105/SMWW.2882.187. [Google Scholar]
- American Public Health Association. 2018b. 9215 Heterotrophic Plate Count. In: Standard Methods For the Examination of Water and Wastewater, 10.2105/smww.2882.188. [DOI] [Google Scholar]
- American Public Health Association. 2018c. 9230 Fecal Enterococcus/Streptococcus groups (2017). In: Standard Methods For the Examination of Water and Wastewater, 7. 10.2105/smww.2882.197. [DOI] [Google Scholar]
- American Public Health Association, 2018d. 9223 Enzyme substrate coliform test (2017). In: Standard Methods For the Examination of Water and Wastewater, 4, 10.2105/smww.2882.194. [DOI] [Google Scholar]
- Aneck-Hahn NH, Van Zijl MC, Swart P, Truebody B, Genthe B, Charmier J, et al. , 2018. Estrogenic activity, selected plasticizers and potential health risks associated with bottled water in South Africa. J. Water Health 16 (2), 253–262. 10.2166/wh.2018.043. [DOI] [PubMed] [Google Scholar]
- Argos M, Kalra T, Rathouz PJ, Chen Y, Pierce B, Parvez F, et al. , 2010. Arsenic exposure from drinking water, and all-cause and chronic-disease mortalities in Bangladesh (HEALS): a prospective cohort study. Lancet 376 (9737), 252–258. 10.1016/S0140-6736(10)60481-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschebrook-Kilfoy B, Heltshe SL, Nuckols JR, Sabra MM, Shuldiner AR, Mitchell BD, et al. , 2012. Modeled nitrate levels in well water supplies and prevalence of abnormal thyroid conditions among the Old Order Amish in Pennsylvania. Environ. Health 11 (1), 6. 10.1186/1476-069X-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball JW, McCleskey RB, 2003. A new cation-exchange method for accurate field speciation of hexavalent chromium. Talanta 61 (3), 305–313. 10.1016/S0039-9140(03)00282-0. [DOI] [PubMed] [Google Scholar]
- Barringer JL, Johnsson PA 1996. Theoretical considerations and a simple method for measuring alkalinity and acidity in low-pH waters by gran titration. U.S. Geological Survey Water-Resources Investigation Report 89–4029. 10.3133/wri894029. [DOI] [Google Scholar]
- Bexfield LM, Toccalino PL, Belitz K, Foreman WT, Furlong ET, 2019. Hormones and Pharmaceuticals in Groundwater Used As a Source of Drinking Water Across the United States. Environ. Sci. Tech 53 (6), 2950–2960. 10.1021/acs.est.8b05592. [DOI] [PubMed] [Google Scholar]
- Bexfield LM, Belitz K, Lindsey BD, Toccalino PL, Nowell LH, 2021. Pesticides and Pesticide Degradates in Groundwater Used for Public Supply across the United States: Occurrence and Human-Health Context. Environ. Sci. Tech 55 (1), 362–372. 10.1021/acs.est.0c05793. [DOI] [PubMed] [Google Scholar]
- Blackwell BR, Ankley GT, Corsi SR, De Cicco LA, Houck KA, Judson RS, et al. , 2017. An“EAR” on environmental surveillance and monitoring: A case study on the use of exposure-activity ratios (EARs) to prioritize sites, chemicals, and bioactivities of concern in Great Lakes waters. Environ. Sci. Tech 51 (15), 8713–8724. 10.1021/acs.est.7b01613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake BE, Fenton SE, 2020. Early life exposure to per- and polyfluoroalkyl substances (PFAS) and latent health outcomes: A review including the placenta as a target tissue and possible driver of peri- and postnatal effects. Toxicology 443, 152565. 10.1016/j.tox.2020.152565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley PM, Journey C, Romanok K, Barber L, Buxton HT, Foreman WT, et al. 2017. Expanded target-chemical analysis reveals extensive mixed-organic-contaminant exposure in USA streams. Environ. Sci. Technol 51 (9), 4792–4802. 10.1021/acs.est.7b00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley PM, Kolpin DW, Romanok KM, Smalling KL, Focazio MJ, Brown JB, et al. , 2018. Reconnaissance of mixed organic and inorganic chemicals in private and public supply tapwaters at selected residential and workplace sites in the United States. Environ. Sci. Tech 52 (23), 13972–13985. 10.1021/acs.est.8b04622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley PM, Journey CA, Berninger JP, Button DT, Clark JM, Corsi SR, et al. 2019. Mixed-chemical exposure and predicted effects potential in wadeable southeastern USA streams. Sci. Total Environ 655, 70–83. 10.1016/j.scitotenv.2018.11.186. [DOI] [PubMed] [Google Scholar]
- Bradley PM, Argos M, Kolpin DW, Meppelink SM, Romanok KM, Smalling KL, et al. , 2020. Mixed organic and inorganic tapwater exposures and potential effects in greater Chicago area, USA. Sci. Total Environ 719, 137236 10.1016/j.scitotenv.2020.137236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley PM, LeBlanc DR, Romanok KM, Smalling KL, Focazio MJ, Cardon MC, et al. , 2021a. Public and private tapwater: Comparative analysis of contaminant exposure and potential risk, Cape Cod, Massachusetts, USA. Environ. Int 152, 106487 10.1016/j.envint.2021.106487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley PM, Padilla IY, Romanok KM, Smalling KL, Focazio MJ, Breitmeyer SE, et al. , 2021b. Pilot-scale expanded assessment of inorganic and organic tapwater exposures and predicted effects in Puerto Rico, USA. Sci. Total Environ 788, 147721 10.1016/j.scitotenv.2021.147721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley PM, Romanok KM, Smalling KL, Focazio MJ, Charboneau R, George CM, et al. , 2022. Tapwater exposures, effects potential, and residential risk management in northern plains nations. Environ. Sci. Technol. Water 2 (10), 1772–1788. 10.1021/acsestwater.2c00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand W, de Jongh CM, van der Linden SC, Mennes W, Puijker LM, van Leeuwen CJ, et al. , 2013. Trigger values for investigation of hormonal activity in drinking water and its sources using CALUX bioassays. Environ. Int 55, 109–118. 10.1016/j.envint.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Breitner LN, Howe KJ, Minakata D, 2019. Effect of Functional Chemistry on the Rejection of Low-Molecular Weight Neutral Organics through Reverse Osmosis Membranes for Potable Reuse. Environ. Sci. Tech 53 (19), 11401–11409. 10.1021/acs.est.9b03856. [DOI] [PubMed] [Google Scholar]
- Brender JD, Weyer PJ, Romitti PA, Mohanty BP, Shinde MU, Vuong AM, et al. , 2013. Prenatal nitrate intake from drinking water and selected birth defects in offspring of participants in the National Birth Defects Prevention Study. Environ. Health Perspect 121 (9), 1083–1089. 10.1289/ehp.1206249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedergreen N, Christensen AM, Kamper A, Kudsk P, Mathiassen SK, Streibig JC, et al. , 2008. A review of independent action compared to concentration addition as reference models for mixtures of compounds with different molecular target sites. Environ. Toxicol. Chem 27 (7), 1621–1632. 10.1897/07-474.1. [DOI] [PubMed] [Google Scholar]
- Childress C, Foreman W, Conner B, Maloney T, 1999. New reporting procedures based on long-term method detection levels and some considerations for interpretations of water-quality data provided by the U.S. Geological Survey National Water Quality Laboratory. U.S. Geological Survey Open-File Report 99–193. 10.3133/ofr99193. [DOI] [Google Scholar]
- Chow SJ, Ojeda N, Jacangelo JG, Schwab KJ, 2021. Detection of ultrashort-chain and other per- and polyfluoroalkyl substances (PFAS) in U.S. bottled water. Water Res. 201, 117292 10.1016/j.watres.2021.117292. [DOI] [PubMed] [Google Scholar]
- Cochrane N, Saranathan S, Morgan M, Dashper S, 2006. Fluoride content of still bottled water in Australia. Aust. Dent. J 51 (3), 242–244. 10.1111/j.1834-7819.2006.tb00436.x. [DOI] [PubMed] [Google Scholar]
- Cohn ML, Waggoner RF, McClatchy JK, 1968. The 7Hll Medium for the Cultivation of Mycobacteria. Am. Rev. Respir. Dis 98 (2), 295–296. https://www.atsjournals.org/doi/pdf/10.1164/arrd.1968.98.2.295. [DOI] [PubMed] [Google Scholar]
- Conley J, Evans N, Cardon M, Rosenblum L, Iwanowicz L, Hartig P, et al. , 2017a. Occurrence and in vitro bioactivity of estrogen, androgen, and glucocorticoid compounds in a nationwide screen of United States stream waters. Environ. Sci. Tech 51 (9), 4781–4791. 10.1021/acs.est.6b06515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley J, Evans N, Mash H, Rosenblum L, Schenck K, Glassmeyer S, et al. , 2017b. Comparison of in vitro estrogenic activity and estrogen concentrations in source and treated waters from 25 US drinking water treatment plants. Sci. Total Environ 579, 1610–1617. 10.1016/j.scitotenv.2016.02.093. [DOI] [PubMed] [Google Scholar]
- Cooper KL, Dashner EJ, Tsosie R, Cho YM, Lewis J, Hudson LG, 2016. Inhibition of poly(ADP-ribose)polymerase-1 and DNA repair by uranium. Toxicol. Appl. Pharmacol 291, 13–20. 10.1016/j.taap.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsi SR, De Cicco LA, Villeneuve DL, Blackwell BR, Fay KA, Ankley GT, et al. , 2019. Prioritizing chemicals of ecological concern in Great Lakes tributaries using high-throughput screening data and adverse outcome pathways. Sci. Total Environ 686, 995–1009. 10.1016/j.scitotenv.2019.05.457. [DOI] [PubMed] [Google Scholar]
- Dai D, Prussin AJ, Marr LC, Vikesland PJ, Edwards MA, Pruden A, 2017. Factors shaping the human exposome in the built environment: opportunities for engineering control. Environ. Sci. Tech 51 (14), 7759–7774. 10.1021/acs.est.7b01097. [DOI] [PubMed] [Google Scholar]
- De Cicco L, Corsi SR, Villeneuve D, Blackwell BR, Ankley GT, 2018. toxEval: Evaluation of measured concentration data using the ToxCast high-throughput screening database or a user-defined set of concentration benchmarks. R package version 1.0.0. https://owi.usgs.gov/R/gran.html [accessed May 1, 2018]. [Google Scholar]
- de Jesus Gaffney V, Almeida CM, Rodrigues A, Ferreira E, Benoliel MJ, Cardoso VV, 2015. Occurrence of pharmaceuticals in a water supply system and related human health risk assessment. Water Res. 72, 199–208. 10.1016/j.watres.2014.10.027. [DOI] [PubMed] [Google Scholar]
- DeSimone LA, McMahon PB, Rosen MR, 2015. The quality of our Nation’s waters: water quality in Principal Aquifers of the United States, 1991–2010. U. S. Geological Survey Circular 1360. Reston, VA, 10.3133/cir1360. [DOI] [Google Scholar]
- Dieter CA, Maupin MA, Caldwell RR, Harris MA, Ivahnenko TI, Lovelace JK, et al. 2018. Estimated use of water in the United States in 2015. U. S. Geological Survey Circular 1441. Reston, VA, 10.3133/cir1441. [DOI] [Google Scholar]
- Dobson CM, 2004. Chemical space and biology. Nature 432 (7019), 824–828. 10.1038/nature03192. [DOI] [PubMed] [Google Scholar]
- Doria d.F.M., 2010. Factors influencing public perception of drinking water quality. Water policy. 12 (1), 1–19. 10.2166/wp.2009.051. [DOI] [Google Scholar]
- Ermler S, Scholze M, Kortenkamp A, 2011. The suitability of concentration addition for predicting the effects of multi-component mixtures of up to 17 anti-androgens with varied structural features in an in vitro AR antagonist assay. Toxicol. Appl. Pharmacol 257 (2), 189–197. 10.1016/j.taap.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Felipe-Sotelo M, Henshall-Bell ER, Evans NDM, Read D, 2015. Comparison of the chemical composition of British and Continental European bottled waters by multivariate analysis. J. Food Compos. Anal 39, 33–42. 10.1016/j.jfca.2014.10.014. [DOI] [Google Scholar]
- Filer DL, Kothiya P, Setzer RW, Judson RS, Martin MT, 2017. tcpl: the ToxCast pipeline for high-throughput screening data. Bioinformatics 33 (4), 618–620. 10.1093/bioinformatics/btw680. [DOI] [PubMed] [Google Scholar]
- Focazio MJ, Tipton D, Dunkle SS, Geiger LH, 2006. The chemical quality of self-supplied domestic well water in the United States. Ground Water Monit. Rem 26 (3), 92–104. 10.1111/j.1745-6592.2006.00089.x. [DOI] [Google Scholar]
- Foreman WT, Williams TL, Furlong ET, Hemmerle DM, Stetson SJ, Jha VK, et al. , 2021. Comparison of detection limits estimated using single- and multi-concentration spike-based and blank-based procedures. Talanta 228, 122139. 10.1016/j.talanta.2021.122139. [DOI] [PubMed] [Google Scholar]
- Furlong E, Noriega M, Kanagy C, Kanagy L, Coffey L, Burkhardt M, 2014. Methods of the National Water Quality Laboratory. Chapter B10. Determination of human-use pharmaceuticals in filtered water by direct aqueous injection–high-performance liquid chromatography/tandem mass spectrometry. U.S. Geological Survey Techniques and Methods. Book 5. Laboratory Analysis. Chap. B10. 10.3133/tm5B10.. [DOI] [Google Scholar]
- García-Esquinas E, Pollán M, Umans JG, Francesconi KA, Goessler W, Guallar E, et al. , 2013. Arsenic exposure and cancer mortality in a US-based prospective cohort: The Strong Heart Study. Cancer Epidemiol. Biomark. Prev 22 (11), 1944–1953. 10.1158/1055-9965.Epi-13-0234-t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellrich V, Brunn H, Stahl T, 2013. Perfluoroalkyl and polyfluoroalkyl substances (PFASs) in mineral water and tap water. J. Environ. Sci. Health A 48 (2), 129–135. 10.1080/10934529.2013.719431. [DOI] [PubMed] [Google Scholar]
- Goumenou M, Tsatsakis A, 2019. Proposing new approaches for the risk characterisation of single chemicals and chemical mixtures: The source related Hazard Quotient (HQS) and Hazard Index (HIS) and the adversity specific Hazard Index (HIA). Toxicol. Rep 6, 632–636. 10.1016/j.toxrep.2019.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JL, Loftin KA, Meyer MT, Ziegler AC, 2010. Cyanotoxin mixtures and taste-and-odor compounds in cyanobacterial blooms from the Midwestern United States. Environ. Sci. Tech 44 (19), 7361–7368. 10.1021/es1008938. [DOI] [PubMed] [Google Scholar]
- Hajna AA, 1955. A new enrichment broth medium for gram-negative organisms of the intestinal group. Public Health Laboratory. 13, 83–89. [Google Scholar]
- Hammer Ø, Harper DA, Ryan PD, 2001. PAST: paleontological statistics software package for education and data analysis. Palaeontol. Electron 4 (1), 9. https://palaeo-electronica.org/2001_1/past/issue1_01.htm. [Google Scholar]
- Hawkins G, 2017. The impacts of bottled water: an analysis of bottled water markets and their interactions with tap water provision. WIREs Water 4 (3), e1203. [Google Scholar]
- Health Canada. 2020. Guidelines for Canadian Drinking Water Quality: Summary Table. https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/ewh-semt/alt_formats/pdf/pubs/water-eau/sum_guide-res_recom/summary-table-EN-2020-02-11.pdf.
- Hergenreder RL, 2011. Trace Metals in Waters by GFAAS, in Accordance with U.S. EPA and Health Canada Requirements. Perkin Elmer Inc, Waltham, MA. [Google Scholar]
- Hladik ML, Focazio MJ, Engle M, 2014. Discharges of produced waters from oil and gas extraction via wastewater treatment plants are sources of disinfection by-products to receiving streams. Sci. Total Environ 466, 1085–1093. 10.1016/j.scitotenv.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Hoffman GL, Fishman MJ, Garbarino JR 1996. Methods of Analysis by the US Geological Survey National Water Quality Laboratory: In-bottle Acid Digestion of Whole-water Samples. U.S. Geological Survey Open-File Report 96–225. US Department of the Interior, US Geological Survey. 10.3133/ofr96225. [DOI] [Google Scholar]
- Horowitz AM, Kleinman DV, Child W, Maybury C, 2015. Perspectives of Maryland Adults Regarding Caries Prevention. Am. J. Public Health 105 (5), e58–e64. 10.2105/ajph.2015.302565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikem A, Odueyungbo S, Egiebor NO, Nyavor K, 2002. Chemical quality of bottled waters from three cities in eastern Alabama. Sci. Total Environ 285 (1), 165–175. 10.1016/S0048-9697(01)00915-9. [DOI] [PubMed] [Google Scholar]
- International Bottled Water Association, 2020. Bottled water code of practice. IBWA, Alexandria, VA: https://bottledwater.org/wp-content/uploads/2020/12/IBWA-MODEL-CODE-2020-Rev-2020-FINAL.pdf. [Google Scholar]
- Javidi A, Pierce G, 2018. US Households’ Perception of Drinking Water as Unsafe and its Consequences: Examining Alternative Choices to the Tap. Water Resour. Res 54 (9), 6100–6113. 10.1029/2017WR022186. [DOI] [Google Scholar]
- Jones RR, Weyer PJ, DellaValle CT, Inoue-Choi M, Anderson KE, Cantor KP, et al. , 2016. Nitrate from drinking water and diet and bladder cancer among postmenopausal women in Iowa. Environ. Health Perspect 124 (11), 1751–1758. 10.1289/EHP191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RR, Weyer PJ, DellaValle CT, Robien K, Cantor KP, Krasner S, et al. , 2017. Ingested nitrate, disinfection by-products, and kidney cancer risk in older women. Epidemiology 28 (5), 703–711. 10.1097/EDE.0000000000000647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobeloch L, Gorski P, Christenson M, Anderson H, 2013. Private Drinking Water Quality in Rural Wisconsin. J. Environ. Health 75 (7), 16–21. www.jstor.org/stable/26329567. [PubMed] [Google Scholar]
- Kohn WG, Maas WR, Malvitz DM, Presson SM, Shaddix KK, 2001. Recommendations for using fluoride to prevent and control dental caries in the United States. https://stacks.cdc.gov/view/cdc/5160.
- Kolpin DW, Hubbard LE, Cwiertny DM, Meppelink SM, Thompson DA, Gray JL, 2021. A comprehensive statewide spatiotemporal stream assessment of per- and polyfluoroalkyl substances (PFAS) in an agricultural region of the United States. Environ. Sci. Technol. Lett 8 (11), 981–988. 10.1021/acs.estlett.1c00750. [DOI] [Google Scholar]
- Kozak NA, Lucas CE, Winchell JM, 2013. Identification of Legionella in the Environment. In: Buchrieser C, Hilbi H (Eds.), Legionella: Methods and Protocols. Humana Press, Totowa, NJ, pp. 3–25. 10.1007/978-1-62703-161-5_1. [DOI] [PubMed] [Google Scholar]
- Krachler M, Shotyk W, 2009. Trace and ultratrace metals in bottled waters: Survey of sources worldwide and comparison with refillable metal bottles. Sci. Total Environ 407 (3), 1089–1096. 10.1016/j.scitotenv.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Kurttio P, Komulainen H, Leino A, Salonen L, Auvinen A, Saha H, 2005. Bone as a possible target of chemical toxicity of natural uranium in drinking water. Environ. Health Perspect 113 (1), 68. 10.1289/ehp.7475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanphear B, Lowry J, Ahdoot S, Baum C, Bernstein A, Bole A, et al. , 2016. Prevention of childhood lead toxicity: Policy statement of the American Academy of Pediatrics Council on Environmental Health. Pediatrics 138 (1), e20161493. [DOI] [PubMed] [Google Scholar]
- Levin MA, Cabelli VJ, 1972. Membrane Filter Technique for Enumeration of Pseudomonas aeruginosa. Appl. Microbiol 24 (6), 864–870. 10.1128/am.24.6.864-870.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley BD, Brewer JH, 1953. The selective antibacterial action of phenylethyl alcohol. J. Am. Pharm. Assoc 42 (1), 6–8. 10.1002/jps.3030420103. [DOI] [PubMed] [Google Scholar]
- Lisle JT, Priscu JC, 2004. The Occurrence of Lysogenic Bacteria and Microbial Aggregates in the Lakes of the McMurdo Dry Valleys, Antarctica. Microb. Ecol 47 (4), 427–439. 10.1007/s00248-003-1007-x. [DOI] [PubMed] [Google Scholar]
- Loftin KA, Graham JL, Hilborn ED, Lehmann SC, Meyer MT, Dietze JE, et al. , 2016. Cyanotoxins in inland lakes of the United States: Occurrence and potential recreational health risks in the EPA National Lakes Assessment 2007. Harmful Algae 56, 77–90. 10.1016/j.hal.2016.04.001. [DOI] [PubMed] [Google Scholar]
- Luo Q, Liu Z-H, Yin H, Dang Z, Wu P-X, Zhu N-W, et al. , 2018. Migration and potential risk of trace phthalates in bottled water: A global situation. Water Res. 147, 362–372. 10.1016/j.watres.2018.10.002. [DOI] [PubMed] [Google Scholar]
- Magdo HS, Forman J, Graber N, Newman B, Klein K, Satlin L, et al. , 2007. Grand rounds: Nephrotoxicity in a young child exposed to uranium from contaminated well water. Environ. Health Perspect 115 (8), 1237. 10.1289/ehp.9707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggioni S, Balaguer P, Chiozzotto C, Benfenati E, 2013. Screening of endocrine-disrupting phenols, herbicides, steroid estrogens, and estrogenicity in drinking water from the waterworks of 35 Italian cities and from PET-bottled mineral water. Environ. Sci. Pollut. Res 20 (3), 1649–1660. 10.1007/s11356-012-1075-x. [DOI] [PubMed] [Google Scholar]
- Marron EL, Mitch WA, von Gunten U, Sedlak DL, 2019. A Tale of Two Treatments: The Multiple Barrier Approach to Removing Chemical Contaminants During Potable Water Reuse. Acc. Chem. Res 52 (3), 615–622. 10.1021/acs.accounts.8b00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleskey RB, Roth DA, Mahony D, Nordstrom DK, Kinsey S, 2019. Sources, fate, and flux of geothermal solutes in the Yellowstone and Gardner Rivers, Yellowstone National Park. WY. Appl. Geochem 111, 104458 10.1016/j.apgeochem.2019.104458. [DOI] [Google Scholar]
- Medlock Kakaley EK, Blackwell BR, Cardon MC, Conley JM, Evans N, Feifarek DJ, et al. , 2020. De facto water reuse: Bioassay suite approach delivers depth and breadth in endocrine active compound detection. Sci. Total Environ 699, 134297 10.1016/j.scitotenv.2019.134297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medlock Kakaley EK, Cardon MC, Evans N, Iwanowicz LR, Allen JM, Wagner E, et al. , 2021. In vitro effects-based method and water quality screening model for use in pre- and post-distribution treated waters. Environ. Sci. Tech 10.1016/j.scitotenv.2020.144750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills K, Falconer S, Cook C, 2010. Fluoride in still bottled water in Australia. Aust. Dent. J 55 (4), 411–416. 10.1111/j.1834-7819.2010.01262.x. [DOI] [PubMed] [Google Scholar]
- Mohammed Abdul KS, Jayasinghe SS, Chandana EPS, Jayasumana C, De Silva PMCS, 2015. Arsenic and human health effects: A review. Environ. Toxicol. Pharmacol 40 (3), 828–846. 10.1016/j.etap.2015.09.016. [DOI] [PubMed] [Google Scholar]
- Moretto A, Bachman A, Boobis A, Solomon KR, Pastoor TP, Wilks MF, et al. , 2017. A framework for cumulative risk assessment in the 21st century. Crit. Rev. Toxicol 47 (2), 85–97. 10.1080/10408444.2016.1211618. [DOI] [PubMed] [Google Scholar]
- Mueller DK, Schertz TL, Martin JD, Sandstrom MW, 2015. Design, analysis, and interpretation of field quality-control data for water-sampling projects. In: U.S. Geological Survey Techniques and Methods Book 4 Chapter C4. 10.3133/tm4C4. [DOI] [Google Scholar]
- National Research Council, 1983. Risk Assessment in the Federal Government: Managing the Process. Washington, DC: The National Academies Press. 10.17226/366, https://www.nap.edu/catalog/366/risk-assessment-in-the-federal-government-managing-the-process. [DOI] [PubMed] [Google Scholar]
- Navas-Acien A, Sharrett AR, Schwartz BS, Guallar E, Silbergeld EK, Nachman KE, et al. , 2005. Arsenic exposure and cardiovascular disease: A systematic review of the epidemiologic evidence. Am. J. Epidemiol 162 (11), 1037–1049. 10.1093/aje/kwi330. [DOI] [PubMed] [Google Scholar]
- Navas-Acien A, Silbergeld EK, Pastor-Barriuso R, Guallar E, 2008. Arsenic exposure and prevalence of type 2 diabetes in US adults. J. Am. Med. Assoc 300 (7), 814–822. 10.1001/jama.300.7.814. [DOI] [PubMed] [Google Scholar]
- Neale P, Leusch F, Escher B, 2021. Bioanalytical Tools in Water Quality Assessment, 2nd Edition:IWA publishing. 10.2166/9781789061987. [DOI] [Google Scholar]
- Neale PA, Escher BI, 2019. In vitro bioassays to assess drinking water quality. Curr. Opin. Environ. Sci. Health 7, 1–7. 10.1016/j.coesh.2018.06.006. [DOI] [Google Scholar]
- New Hampshire Department of Environmental Services, 2021. Arsenic in New Hampshire Well Water. https://www.des.nh.gov/sites/g/files/ehbemt341/files/documents/2020-01/dwgb-3-2.pdf.
- Norman JE, Toccalino PL, Morman SA, 2018. Health-Based Screening Levels for evaluating water-quality data (2nd ed.). Available at: 10.5066/F71C1TWP [accessed February 10, 2020]. [DOI] [Google Scholar]
- Norton SB, Rodier DJ, van der Schalie WH, Wood WP, Slimak MW, Gentile JH, 1992. A framework for ecological risk assessment at the EPA. Environ. Toxicol. Chem 11 (12), 1663–1672. 10.1002/etc.5620111202. [DOI] [Google Scholar]
- Olson ED, Poling D, Solomon G 1999. Bottled water: pure drink or pure hype?: National Resources Defense Council. https://www.nrdc.org/sites/default/files/bottled-water-pure-drink-or-pure-hype-report.pdf. [Google Scholar]
- Pape AD, Seo M, 2015. Reports of Water Quality Violations Induce Consumers to Buy Bottled Water. Agric. Resour. Econ. Rev 44 (1), 78–93. 10.1017/S1068280500004639. [DOI] [Google Scholar]
- Paul FK, Gagne M, Loo L-H, Karamertzanis P, Netzeva T, Sobanski T, et al. , 2020. Utility of in vitro bioactivity as a lower bound estimate of in vivo adverse effect levels and in risk-based prioritization. Toxicol. Sci 173 (1), 202–225. 10.1093/toxsci/kfz201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce RG, Setzer RW, Strope CL, Sipes NS, Wambaugh JF, 2017. httk: R Package for High-Throughput Toxicokinetics. J. Stat. Softw 79 (4), 1–26. 10.18637/jss.v079.i04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrisek R, Hall J, 2017. Evaluation of a most probable number method for the enumeration of Legionella pneumophila from North American potable and nonpotable water samples. J. Water Health 16 (1), 25–33. 10.2166/wh.2017.118. [DOI] [PubMed] [Google Scholar]
- Pichler G, Grau-Perez M, Tellez-Plaza M, Umans J, Best L, Cole S, et al. , 2019. Association of arsenic exposure with cardiac geometry and left ventricular function in young adults. Circulation. Cardiovascular Imaging 12 (5), e009018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce G, Gonzalez S, 2017. Mistrust at the tap? Factors contributing to public drinking water (mis) perception across US households. Water Policy 19 (1), 1–12. 10.2166/wp.2016.143. [DOI] [Google Scholar]
- Postma J, Butterfield PW, Odom-Maryon T, Hill W, Butterfield PG, 2011. Rural children’s exposure to well water contaminants: Implications in light of the American Academy of Pediatrics’ recent policy statement. J. Am. Acad. Nurse Pract 23 (5), 258–265. 10.1111/j.1745-7599.2011.00609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quist AJL, Inoue-Choi M, Weyer PJ, Anderson KE, Cantor KP, Krasner S, et al. , 2018. Ingested nitrate and nitrite, disinfection by-products, and pancreatic cancer risk in postmenopausal women. Int. J. Cancer 142 (2), 251–261. 10.1002/ijc.31055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. 2019. R: A Language and Environment for Statistical Computing. Version 3.5.2. Vienna Austria:R Foundation for Statistical Computing, https://www.R-project.org. [Google Scholar]
- Raymond-Whish S, Mayer LP, O’Neal T, Martinez A, Sellers MA, Christian PJ, et al. , 2007. Drinking water with uranium below the US EPA water standard causes estrogen receptor–dependent responses in female mice. Environ. Health Perspect 115 (12), 1711. 10.1289/ehp.9910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Real M, Molina-Molina J-M, Jiménez-Díaz I, Arrebola JP, Sáenz J-M, Fernández MF, et al. , 2015. Screening of hormone-like activities in bottled waters available in Southern Spain using receptor-specific bioassays. Environ. Int 74, 125–135. 10.1016/j.envint.2014.10.006. [DOI] [PubMed] [Google Scholar]
- Richard AM, Judson RS, Houck KA, Grulke CM, Volarath P, Thillainadarajah I, et al. , 2016. ToxCast chemical landscape: paving the road to 21st century toxicology. Chem. Res. Toxicol 29 (8), 1225–1251. 10.1021/acs.chemrestox.6b00135. [DOI] [PubMed] [Google Scholar]
- Rodwan JG 2021. Bottled water 2020: Continued upward movement U.S. and International Developments and Statistics. Bottled Water Reporter. 10–19. https://bottledwater.org/wp-content/uploads/2021/07/2020BWstats_BMC_pub2021BWR.pdf. [Google Scholar]
- Rogan WJ, Brady MT, 2009. Drinking water from private wells and risks to children. Pediatrics 123 (6), e1123–e1137. 10.1542/peds.2009-0752. [DOI] [PubMed] [Google Scholar]
- Romanok KM, Kolpin DW, Meppelink SM, Argos M, Brown J, DeVito M, et al. , 2018. Methods used for the collection and analysis of chemical and biological data for the Tapwater Exposure Study, United States, 2016–17. U.S. Geological Survey Open-File Report 2018–1098. Reston, VA. 10.3133/ofr20181098. [DOI] [Google Scholar]
- Romanok KM, Bradley PM, Smalling KL, Hladik ML, Gray JL, Kanagy LK, McCleskey RB, Roth DA, Lisle JT, Givens CE. 2022. Target-Chemical Concentrations and Microbiological Results for Assessment of Mixed Contaminant and Biological Exposures in Bottled Water, 2020. U.S. Geological Survey Data Release. 10.5066/P97K1I4L. [DOI] [Google Scholar]
- Rose D, Sandstrom M, Murtagh L, 2016. Methods of the National Water Quality Laboratory. Chapter B12. Determination of heat purgeable and ambient purgeable volatile organic compounds in water by gas chromatography/mass spectrometry. U. S. Geological Survey Techniques and Methods. Book 5. Laboratory Analysis. 10.3133/tm5B12. [DOI] [Google Scholar]
- Saleh MA, Abdel-Rahman FH, Woodard BB, Clark S, Wallace C, Aboaba A, et al. , 2008. Chemical, microbial and physical evaluation of commercial bottled waters in greater Houston area of Texas. J. Environ. Sci. Health A 43 (4), 335–347. 10.1080/10934520701795400. [DOI] [PubMed] [Google Scholar]
- Sandstrom MW, Kanagy LK, Anderson CA, Kanagy CJ, 2015. Methods of the National Water Quality Laboratory. Chapter B11. Determination of pesticides and pesticide degradates in filtered water by direct aqueous-injection liquid chromatography-tandem mass spectrometry. U.S. Geological Survey Techniques and Methods. Book 5. Laboratory Analysis. 10.3133/tm5B11. [DOI] [Google Scholar]
- Schroeder AL, Ankley GT, Houck KA, Villeneuve DL, 2016. Environmental surveillance and monitoring—The next frontiers for high-throughput toxicology. Environ. Toxicol. Chem 35 (3), 513–525. 10.1002/etc.3309. [DOI] [PubMed] [Google Scholar]
- Seldén AI, Lundholm C, Edlund B, Högdahl C, Ek B-M, Bergström BE, et al. , 2009. Nephrotoxicity of uranium in drinking water from private drilled wells. Environ. Res 109 (4), 486–494. 10.1016/j.envres.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Shih Y-H, Islam T, Hore SK, Sarwar G, Shahriar MH, Yunus M, et al. , 2017. Associations between prenatal arsenic exposure with adverse pregnancy outcome and child mortality. Environ. Res 158, 456–461. 10.1016/j.envres.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AH, Steinmaus CM, 2009. Health effects of arsenic and chromium in drinking water: recent human findings. Annu. Rev. Public Health 30 (1), 107–122. 10.1146/annurev.publhealth.031308.100143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalter D, O’Malley E, von Gunten U, Escher BI, 2020. Mixture effects of drinking water disinfection by-products: implications for risk assessment. Environ. Sci. Water Res. Technol 6, 2341–2351. 10.1039/C9EW00988D. [DOI] [Google Scholar]
- Stanhope J, McAuley K, Cook A, Weinstein P, 2020. Estimating Trihalomethane Concentrations in Bottled Spring Water. Exposure and Health. 12 (4), 877–881. 10.1007/s12403-020-00350-z. [DOI] [Google Scholar]
- State of New Jersey, 2021. New Jersey Administrative Code. 7:10: Safe Drinking Water Act Rules. https://www.nj.gov/dep/rules/rules/njac7_10.pdf. [Google Scholar]
- Toccalino PL, Norman JE, Scott JC, 2012. Chemical mixtures in untreated water from public-supply wells in the US—occurrence, composition, and potential toxicity. Sci. Total Environ 431, 262–270. 10.1016/j.scitotenv.2012.05.044. [DOI] [PubMed] [Google Scholar]
- Triantafyllidou S, Edwards M, 2012. Lead (Pb) in tap water and in blood: implications for lead exposure in the United States. Crit. Rev. Environ. Sci. Technol 42 (13), 1297–1352. 10.1080/10643389.2011.556556. [DOI] [Google Scholar]
- Tröger R, Klöckner P, Ahrens L, Wiberg K, 2018. Micropollutants in drinking water from source to tap - Method development and application of a multiresidue screening method. Sci. Total Environ 627, 1404–1432. 10.1016/j.scitotenv.2018.01.277. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency. 1997. Method 300.1: Determination of inorganic anions in drinking Water by ion chromatography, Revision 1.0. U.S. Environmental Protection Agency, https://www.epa.gov/sites/default/files/2015-06/documents/epa-300.1.pdf. [Google Scholar]
- U.S. Environmental Protection Agency. 2011. EPA’s National-scale Air Toxics Assessment, An Overview of Methods for EPA’s National-Scale Air Toxics Assessment. https://www.epa.gov/sites/production/files/2015-10/documents/2005-nata-tmd.pdf.
- U.S. Environmental Protection Agency. 2012. Sustainable Futures / Pollution Prevention (P2) Framework Manual. EPA-748-B12-001. Washington, D.C.:U.S. Environmental Protection Agency, https://www.epa.gov/sustainable-futures/sustainable-futures-p2-framework-manual. [Google Scholar]
- U.S. Environmental Protection Agency. 2014. Inductively coupled plasma-optical emission spectrometry, Method 6010D. EPA SW-846 Update V, accessed November 2, 2017. https://www.epa.gov/sites/production/files/2015-12/documents/6010d.pdf.
- U.S. Environmental Protection Agency. 2017. 40 C.F.R. § 131: Water Quality Standards. https://www.ecfr.gov/cgi-bin/text-idx?SID=454a7b51118b27f20cef29ff071c1440&node=40:22.0.1.1.18&rgn=div5.
- U.S. Environmental Protection Agency. 2018. 2018 Edition of the Drinking Water Standards and Health Advisories. EPA 822-F-18-001. https://www.epa.gov/sites/production/files/2018-03/documents/dwtable2018.pdf.
- U.S. Environmental Protection Agency. 2020a. EPA’s National Center for Computational Toxicology: ToxCast database (invitroDB) vers. 5.0. Available at: 10.23645/epacomptox.6062623.v5. [DOI] [Google Scholar]
- U.S. Environmental Protection Agency. 2020b. 40 C.F.R. § 136: Guidelines establishing test procedures for the analysis of pollutants. http://www.ecfr.gov/cgi-bin/text-idx?SID=3c78b6c8952e5e79268e429ed98bad84&mc=true&node=pt40.23.136&rgn=div5.
- U.S. Environmental Protection Agency. 2021a. 40 C.F.R. § 141: National Primary Drinking Water Regulations. https://www.ecfr.gov/cgi-bin/retrieveECFR?gp=1&SID=339dba02478821f8412c44292656bd34&ty=HTML&h=L&mc=true&n=pt40.25.141&r=PART#_top.
- U.S. Environmental Protection Agency. 2021b. Private drinking water wells. Available at: https://www.epa.gov/privatewells [accessed March 30, 2021].
- U.S. Environmental Protection Agency. 2021c. 42 U.S.C. § 300g-6: Prohibition on the use of lead pipes, solder, and flux. https://www.govinfo.gov/content/pkg/USCODE-2010-title42/pdf/USCODE-2010-title42-chap6A-subchapXII-partB-sec300g-6.pdf.
- U.S. Environmental Protection Agency. 2021d. How EPA Regulates Drinking Water Contaminants. Available at: https://www.epa.gov/dwregdev/how-epa-regulates-drinking-water-contaminants [accessed July 11, 2021].
- U.S. Environmental Protection Agency. 2021e. National Primary Drinking Water Regulations. Available at: https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations [accessed July 11, 2021].
- U.S. Environmental Protection Agency National Center for Computational Toxicology. 2020. ToxCast Database InvitroDB3.2. Available at: 10.23645/epacomptox.6062623.v4. [DOI] [Google Scholar]
- U.S. Food & Drug Administration. 2021. 21 C.F.R. § 165.110: Bottled water. https://www.ecfr.gov/current/title-21/chapter-I/subchapter-B/part-165/subpart-B/section-165.110#page-top.
- U.S. Geological Survey. variously dated. National field manual for the collection of water-quality data. U.S. Geological Survey Techniques of Water-Resources Investigations, book 9, chaps A1–A10. Reston, VA. 10.3133/fs20103121. [DOI] [Google Scholar]
- U.S. Public Health Service, 2015. U.S. Public Health Service recommendation for fluoride concentration in drinking water for the prevention of dental caries. Public Health Rep. 130 (4), 318–331. 10.1177/003335491513000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zijl MC, Aneck-Hahn NH, Swart P, Hayward S, Genthe B, De Jager C, 2017. Estrogenic activity, chemical levels and health risk assessment of municipal distribution point water from Pretoria and Cape Town, South Africa. Chemosphere 186, 305–313. 10.1016/j.chemosphere.2017.07.130. [DOI] [PubMed] [Google Scholar]
- Villanueva CM, Kogevinas M, Cordier S, Templeton MR, Vermeulen R, Nuckols JR, et al. , 2014. Assessing exposure and health consequences of chemicals in drinking water: current state of knowledge and research needs. Environ. Health Perspect 122 (3), 213. 10.1289/ehp.1206229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M, Oehlmann J, 2009. Endocrine disruptors in bottled mineral water: total estrogenic burden and migration from plastic bottles. Environ. Sci. Pollut. Res 16 (3), 278–286. 10.1007/s11356-009-0107-7. [DOI] [PubMed] [Google Scholar]
- Wang C, Ye D, Li X, Jia Y, Zhao L, Liu S, et al. , 2021. Occurrence of pharmaceuticals and personal care products in bottled water and assessment of the associated risks. Environ. Int 155, 106651 10.1016/j.envint.2021.106651. [DOI] [PubMed] [Google Scholar]
- Wang Z, Walker GW, Muir DCG, Nagatani-Yoshida K, 2020. Toward a global understanding of chemical pollution: a first comprehensive analysis of national and regional chemical inventories. Environ. Sci. Tech 54 (5), 2575–2584. 10.1021/acs.est.9b06379. [DOI] [PubMed] [Google Scholar]
- Ward MH, deKok TM, Levallois P, Brender J, Gulis G, Nolan BT, et al. , 2005. Workgroup report: Drinking-water nitrate and health - recent findings and research needs. Environ. Health Perspect 113 (11), 1607–1614. 10.1289/ehp.8043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward MH, Jones RR, Brender JD, De Kok TM, Weyer PJ, Nolan BT, et al. , 2018. Drinking water nitrate and human health: an updated review. Int. J. Environ. Res. Public Health 15 (7), 1557. 10.3390/ijerph15071557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson VS, Bobseine K, Gray LE, 2004. Development and characterization of a cell line that stably expresses an estrogen-responsive luciferase reporter for the detection of estrogen receptor agonist and antagonists. Toxicol. Sci 81 (1), 69–77. 10.1093/toxsci/kfh180. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO). 2011. Guidelines for drinking-water quality, Fourth edition incorporating the first addendum. https://apps.who.int/iris/rest/bitstreams/1080656/retrieve. [PubMed]
- Zivin JG, Neidell M, Schlenker W, 2011. Water Quality Violations and Avoidance Behavior: Evidence from Bottled Water Consumption. Am. Econ. Rev 101 (3), 448–453. 10.1257/aer.101.3.448. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in the supporting information file and in the cited Romanok et al. 2022 USGS Data Release.


