Abstract
Background
In the absence of evidence-based strategies to improve patient outcomes, the management of patients with severe idiopathic pulmonary fibrosis (IPF) exacerbations may vary widely across centres. We assessed between-hospital variation in practices and mortality for patients with severe IPF exacerbations.
Methods
Using the Premier Healthcare Database from 1 October 2015 to 31 December 2020, we identified patients admitted to intensive care unit (ICU) or intermediate care unit with an IPF exacerbation. We assessed idiosyncratic, between-hospital variation in ICU practices (invasive mechanical ventilation (IMV), non-invasive mechanical ventilation (NIMV), corticosteroid use, and immunosuppressive and/or antioxidant use) and hospital mortality by determining median risk-adjusted hospital rates and intraclass correlation coefficients (ICCs) from hierarchical multivariable regression models. A priori, an ICC>15% was deemed ‘high variation’.
Results
We identified 5256 critically ill patients with a severe IPF exacerbation at 385 US hospitals. Hospital median risk-adjusted rates of practices were: IMV (14% (IQR: 8.3%–26%)), NIMV (42% (31%–54%)), corticosteroid use (89% (84%–93%)), and immunosuppressive and/or antioxidant use (3.3% (1.9%–5.8%)). Model ICCs were: IMV (19% (95% CI: 18% to 21%)), NIMV (15% (13% to 16%)), corticosteroid use (9.8% (8.3% to 11%)), and immunosuppressive and/or antioxidant use (8.5% (7.1% to 9.9%)). The median risk-adjusted hospital mortality was 16% (IQR: 11%–24%) with an ICC of 7.5% (95% CI: 6.2% to 8.9%).
Interpretation
We observed high variation in the use of IMV and NIMV, and less variation in corticosteroid and immunosuppressant and/or antioxidant use among patients hospitalised with severe IPF exacerbations. Further research is needed to guide the decisions surrounding initiation of IMV and role of NIMV and to understand the effectiveness of corticosteroids among patients with severe IPF exacerbations.
Keywords: Critical Care, Interstitial Fibrosis
WHAT IS ALREADY KNOWN ON THIS TOPIC
Patients admitted with severe exacerbations of idiopathic pulmonary fibrosis (IPF) have poor outcomes. Optimal treatment strategies, including use of invasive mechanical ventilation (IMV), non-invasive mechanical ventilation (NIMV), corticosteroids and immunosuppressant and/or antioxidants, are unclear and may vary widely.
WHAT THIS STUDY ADDS
We observed high variation in the use of IMV and less variation in corticosteroid and immunosuppressant and/or antioxidant use among patients hospitalised with severe IPF exacerbations.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
High variation in use of IMV and NIMV suggests significant clinical uncertainty in the optimal use of IMV and NIMV among patients with severe exacerbations of IPF. Further, in light of near ubiquitous corticosteroid use, further work is needed to understand the effectiveness of corticosteroid use among patients with severe IPF exacerbations.
Introduction
Idiopathic pulmonary fibrosis (IPF), the most common idiopathic interstitial lung disease (ILD), leads to chronic, progressive fibrosis of the lung parenchyma.1 Around 10% of patients with IPF are hospitalised yearly for acute exacerbations triggered by infections, aspirations, drug toxicities or idiopathic causes, and some require critical care within an intensive care unit (ICU). Outcomes for patients with severe IPF exacerbations are poor with ICU mortality rates around 20%–30%2–4; the contributions of various practices to patient outcomes remain unclear.
Therapeutic options for acute exacerbations of IPF are limited. Joint-society practice guidelines issue a weak recommendation against the use of invasive mechanical ventilation (IMV) for a majority of patients based on small, older studies demonstrating mortality as high as 90% in patients who received IMV.5–7 Non-invasive mechanical ventilation (NIMV), on the other hand, is noted to be appropriate in some patients, but little additional guidance is given regarding its use.5 Guidelines also weakly recommend corticosteroids, although the benefit versus risk of these agents is debated.5 7 Multiple non-steroidal immunomodulatory agents have been assessed for treatment of IPF exacerbations, with some trials showing worse outcomes with the addition of these therapies (eg, cyclophosphamide) and only small studies demonstrating efficacy of other agents in the acute setting (eg, rituximab).8–11 Although lung transplantation may offer improved survival for select patients with IPF, transplants are unavailable to the majority of patients.12 13 As a result of both gaps in evidence and lack of treatment options for IPF, practice patterns and outcomes among patients hospitalised with severe IPF exacerbations are not well studied. Thus, we assessed hospital-level variation in practices and outcomes for critically ill patients with IPF in recent years.
Methods
Data source and study cohort
We used the Premier Healthcare Database,14 containing enhanced multicentre claims data from ~25% of US hospitalisations, to identify adult patients hospitalised with severe IPF exacerbations from 1 October 2015 to 31 December 2020. We defined severe IPF exacerbations as patients who had at least one ICU or intermediate care unit (ie, step-down unit) encounter during a hospital admission with either (a) an International Classification of Diseases, Tenth Revision (ICD-10) diagnosis code for IPF (J84.111, J84.112)2 as primary or admitting diagnosis or (b) acute or chronic respiratory failure, pneumonia or ARDS (J96.x, J09.x, J10.x, J11.x, J12.x, J13.x, J14.x, J15.x, J16.x, J17.x, J18.x, J22.x, J80 or R0603) as primary or admitting diagnosis with an IPF ICD-10 code as a secondary diagnosis. In sensitivity analysis, the cohort was restricted to patients with only IPF as a primary or admitting diagnosis (ICD-10 J84.111, J84.112).2
Patients were excluded if they: had an ICD-10 code for any alternate cause of ILD (eg, idiopathic non-specific interstitial pneumonitis, cryptogenic organising pneumonia) or systemic conditions that could be associated with an alternate ILD (eg, rheumatoid arthritis, systemic sclerosis) during index hospitalisation,2 were <50 years of age (low likelihood of having true IPF),1 had an ICD-10 code for chronic obstructive pulmonary disease as a primary or admitting diagnosis (to ensure COPD exacerbation was not primary process), had an ICD-10 code for congestive heart failure as a primary or admitting diagnosis (per revised acute exacerbation of IPF diagnostic criteria in 2016),8 were transferred from another hospital or had a lung transplant surgery within the first 2 days of admission (as they may have been admitted for an immediate and available lung transplant). For patients with multiple hospitalisations meeting inclusion criteria, we randomly selected one hospitalisation for inclusion in the study. Lastly, we excluded patients who were admitted to hospitals with <5 patients meeting inclusion criteria in order to increase the likelihood that our models yielded stable estimates. ICD-10 and charge codes used for inclusion and exclusion criteria can be found in online supplemental appendix 1.
bmjresp-2022-001593supp001.pdf (214.8KB, pdf)
Outcomes
We assessed risk-adjusted rates and between-hospital variation in four practices (a) IMV use, (b) NIMV use (c) corticosteroid use (excluding patients who received transplants) and (d) non-corticosteroid immunosuppressive and/or antioxidant use (azathioprine, methotrexate, mycophenolate, rituximab, cyclophosphamide, N-acetylcysteine) (excluding patients who received transplants). We also assessed risk-adjusted rates and between-hospital variation in (a) mortality among all patients, (b) a composite of mortality or discharge to hospice among all patients and (c) mortality among patients who received IMV (online supplemental appendix 1).
Statistical analysis
We report means (SD), medians (IQR) and proportions (%) where appropriate to summarise patient-level and hospital-level covariables.
We characterised between-hospital variation in mortality and practices using hierarchical multivariable logistic regression models with hospital of admission as a random effect. Models were adjusted for patient-level and hospital-level covariables as fixed effects. Patient-level covariables included age, sex, race and ethnicity, year of discharge, admission source, insurance provider, prior IPF admission (within premier-contributing hospitals), comorbidities present on admission (via validated algorithm merging the Charlson and Elixhauser scores),15 acute organ dysfunction present on admission16 17 and IMV on admission (not adjusted for in models for IMV and NIMV). Hospital-level covariables included US census region,14 urban or rural location,14 safety net status (hospitals in the highest quartile of each region, ranked by proportion of hospitalised patients who are uninsured or on Medicaid),18–20 hospital bed size, hospital teaching status and transplant centre status (hospitals that had billed for lung transplantation during the years of the study). ICD-10 and charge codes used to define covariables can be found in online supplemental appendix 1.
From each model, we identified (a) the median hospital risk-adjusted rate of each outcome with 95% CIs calculated by 1000-fold bootstrapping, (b) the intraclass correlation coefficient (ICC, the proportion of variance attributable to between-hospital variance) and (c) the adjusted association between covariables and each outcome21 (online supplemental appendix 2). An ICC of 15% or greater was considered ‘high’ variation between hospitals, as has been described in other health services utilisation studies.22 In sensitivity analysis, analogous models were built for the subgroup of patients with only IPF as a primary or admitting diagnosis (ICD-10 J84.111, J84.112).2
As an exploratory analysis to assess the degree to which between-hospital variation in mortality was explained by hospital practices, we calculated the proportional change in variance for each practice (the % change in variance attributable to the hospital random effect observed after adding hospital rates of each practice individually to the mortality model, online supplemental appendix 2).23
R (V.4.0.2) was used for analyses. Hypothesis tests were two-sided and alpha was set at 0.05.
Patient and public involvement
It was not appropriate to involve patients or the public in the design, conduct, reporting, or dissemination plans of our research.
Results
Patient and hospital characteristics
We identified 5256 critically ill patients with a severe IPF exacerbation meeting inclusion criteria at 385 unique hospitals from 1 October 2015 to 1 December 2020 (online supplemental appendix 3). The average age of patients was 73.6 years (SD 9.3 years). The majority of patients were men (62%) and white (82%). Most patients were admitted from home (87%) and had Medicare insurance (80%) (table 1). Hospitals were mostly urban (88%); 152 (39%) were teaching hospitals and 188 (49%) were in the South (table 2).
Table 1.
Baseline characteristics of patients admitted with severe IPF exacerbations
| All (n=5256) | |
| Age—years ±SD | 73.6 ±9.3 |
| Male, n (%) | 3267 (62) |
| Race, n (%) | |
| White | 4328 (82) |
| Black | 335 (6.4) |
| Asian | 147 (2.8) |
| Other | 330 (6.3) |
| Unknown | 116 (2.2) |
| Hispanic, n (%) | 426 (8.1) |
| Admission source, n (%) | |
| Home | 4559 (87) |
| Clinic | 501 (9.5) |
| Other | 196 (3.7) |
| Insurance status, n (%) | |
| Medicaid/uninsured | 285 (5.4) |
| Medicare | 4204 (80) |
| Private | 635 (12.1) |
| Acute organ dysfunction, n (%)* | |
| Cardiovascular | 443 (8.4) |
| Respiratory | 951 (18.1) |
| Neurologic | 260 (4.9) |
| Haematologic | 302 (5.7) |
| Hepatic | 14 (0.3) |
| Renal | 788 (15) |
| Comorbidities, n (%)* | |
| Alcohol abuse | 84 (1.6) |
| Any tumour | 360 (6.8) |
| Cardiac arrhythmias | 1788 (34) |
| Chronic pulmonary disease | 3531 (67) |
| Coagulopathy | 362 (6.9) |
| Complicated diabetes | 1047 (20) |
| Congestive heart failure | 2142 (41) |
| Deficiency anaemia | 830 (16) |
| Dementia | 281 (5.3) |
| Fluid and electrolyte disorder | 1891 (36) |
| HIV/AIDS | 9 (0.2) |
| Hemiplegia | 15 (0.3) |
| Hypertension | 3768 (72) |
| Liver disease | 199 (3.8) |
| Metastatic cancer | 119 (2.3) |
| Peripheral vascular disease | 452 (8.6) |
| Psychosis | 853 (16) |
| Pulmonary circulation disorder | 1972 (35) |
| Renal failure | 1093 (21) |
| Weight loss | 729 (14) |
| Mechanically ventilated within first 24 hours of admission, n (%) | 457 (8.4) |
| Prior IPF hospitalisation, n (%) | 154 (2.9) |
*Acute organ dysfunction and comorbidities identified by ICD-10 codes present on admission (online supplemental appendix 1).
IPF, idiopathic pulmonary fibrosis.
Table 2.
Baseline hospital characteristics
| Hospitals, n, (n=385) | |
| Urban, n % | 337 (88) |
| Safety net status, n % | 95 (25) |
| Bed size, n % | |
| >500 | 101 (26) |
| 400–499 | 41 (11) |
| 300–399 | 72 (19) |
| 200–299 | 86 (22) |
| 0–199 | 85 (22) |
| Teaching status, n % | 152 (39) |
| Geographic region, n % | |
| South | 188 (49) |
| West | 63 (16) |
| Northeast | 50 (13) |
| Midwest | 84 (22) |
| Transplant centre, n % | 10 (2.6) |
Hospital-level variation in practices
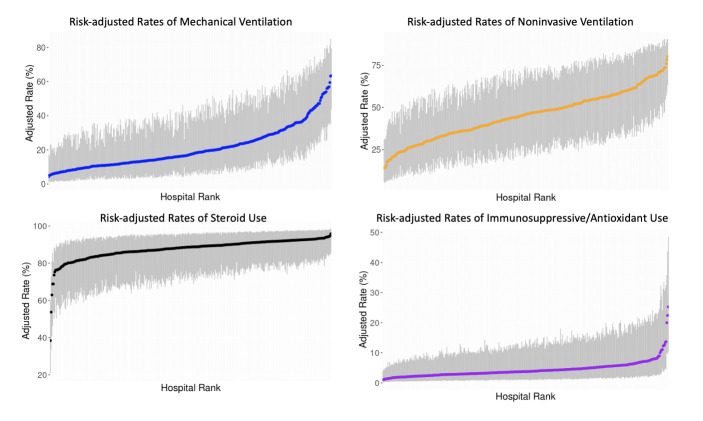
Among critically ill patients with a severe IPF exacerbation, 1082 (21%) received IMV, and the median risk-adjusted hospital IMV rate was 14% (IQR: 8.3%–26%). We observed an ICC for IMV of 19% (95% CI: 18 to 21%). There were 2271 patients (43%) who received NIMV and the median risk-adjusted hospital IMV rate was 42% (IQR: 31%–54%). We observed an ICC for NIMV of 15% (95% CI: 13% to 16%). There were 629 patients (12%) who received both IMV and NIMV. After excluding the 39 patients who received a lung transplant, 4504 (86%) of 5217 patients received corticosteroids and 270 (5.2%) received other immunosuppressives and/or antioxidant therapy; median risk-adjusted hospital rates were 89% (IQR: 84%–93%) and 3.3% (IQR: 1.9%–5.8%), respectively. We observed an ICC for corticosteroid use of 9.8% (8.3%–11%), and an ICC for immunosuppressive and/or antioxidant use of 8.5% (7.1%–9.9%). Risk-adjusted rates of IMV, NIMV, corticosteroid and immunosuppressive/antioxidant use for each hospital from lowest to highest are depicted in figure 1. Adjusted ORs for covariables and practices are included in the appendix (online supplemental appendix 4). Sensitivity analysis with patients with IPF as primary diagnosis codes yielded similar results (online supplemental appendix 5).
Figure 1.
Risk-adjusted rates of practices for patients with severe exacerbations of idiopathic pulmonary fibrosis (IPF). Hospital rates of practices for all patients and patients who received invasive mechanical ventilation, non-invasive mechanical ventilation, corticosteroid and immunosuppressive/antioxidant therapy. Blue, orange, black and purple dots (and associated 95% confidence intervals in grey) show the proportion of patients with severe IPF exacerbations who received each intervention at each hospital.
Hospital-level variation in mortality
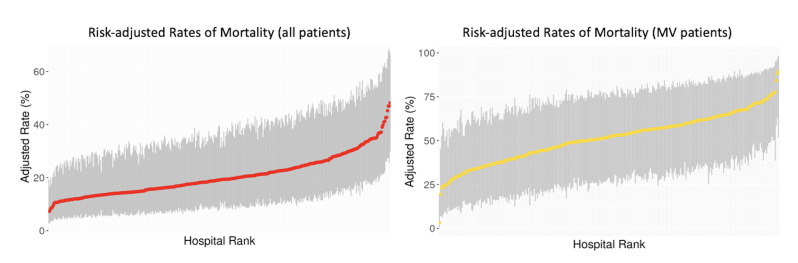
During hospitalisation, 1048 (20%) of all patients died; the median risk-adjusted hospital mortality rate was 16% (IQR: 11%–24%) (figure 2) and the ICC was 7.5% (95% CI: 6.2% to 8.9%) for mortality. The median risk-adjusted rate of hospital mortality or discharge to hospice was 32% (IQR: 23–43%) and the ICC was 4.6% (95% CI: 3.5% to 5.7%).
Figure 2.
Risk-adjusted rates of mortality for patients with severe exacerbations of idiopathic pulmonary fibrosis (IPF). Hospital rates of mortality for all patients and patients who received invasive mechanical ventilation (MV. Red and yellow dots (and associated 95% CIs in grey) show the proportion of patients with severe IPF exacerbations who died at each hospital.
Among the 1082 patients who received IMV, 523 (48%) died during hospitalisation. The median risk-adjusted hospital mortality rate for patients who received IMV was 48% (IQR: 35%–60%) (figure 2) and the ICC for mortality was 6.4% (95% CI: 5.1% to 7.7%). Among patients receiving IMV, admission to a lung transplant centre was associated with lower mortality (aOR: 0.43 (95% CI: 0.22 to 0.85)); 37 out of 84 patients (44%) who received IMV at lung transplant centres received a lung transplant during admission. Adjusted ORs for covariables and mortality among the full cohort and among patients receiving IMV are included in the appendix (online supplemental appendix 2). Sensitivity analysis with patients with IPF as primary diagnosis codes yielded similar results (online supplemental appendix 5).
Relationship between practice patterns and mortality
After adding hospital-level rates of IMV to the mortality model, the ICC decreased from 7.5% to 6.9% (95% CI: 5.6% to 8.1%) for a proportional change in variance of 8.0%. Adding hospital-level rates of NIMV to the mortality model decreased the ICC from 7.5% to 6.4% (95% CI: 5.1% to 7.6%) for a proportional change in variance of 15%. Adding corticosteroid use and immunosuppressive/antioxidant therapy to mortality models changed the ICC to 7.2% (5.9%–8.5%) and 7.6% (6.3%–9.0%) with proportional change in variance of 4.0% and −1.3%, respectively.
Discussion
We examined variation in practices and mortality for patients hospitalised with severe IPF exacerbations across the USA from October 2015 to December 2020. We observed high variation in the use of IMV and NIMV for patients with severe IPF, and less variation in use of corticosteroids and immunosuppressive/antioxidant agents between hospitals. As variation in IMV and NIMV use also partially accounted for the observed variation in mortality, our results highlight the critical need for further data guiding the decision to use IMV and NIMV in patients with severe IPF exacerbations.
The most recent ATS guidelines from 2011 issue a weak recommendation against the use of IMV in patients with IPF based on older studies estimating ~90% hospital mortality in patients with IPF who received IMV.5 More recent studies have demonstrated mortality rates around 50% in these patients,4 6 8 24 25 which is comparable to our observed median hospital mortality rate among patients receiving IMV of 48% (IQR: 35%–60%). The discrepancy between guidelines based on older data with more recent clinical experiences may contribute to significant clinical uncertainty in whether to use NIMV or IMV for severe IPF exacerbations, as illustrated by our findings of high, idiosyncratic hospital practice variation in NIMV and MV use.5
Similarly, despite only weak guideline recommendation to treat acute exacerbations of IPF with corticosteroids,5 corticosteroids were used in the vast majority of patients with severe IPF exacerbations. Reasons underlying such high use may be related to a lack of other evidence-based therapeutic options and the belief that there is an increased inflammatory response in acute exacerbations of IPF.26 Nevertheless, there remains significant debate among experts as to whether corticosteroids may have adverse immune effects on the underlying fibrotic process in IPF, and studies have demonstrated that their use may actually contribute to worse outcomes during an exacerbation.27 28 Future studies assessing the clinical benefit of corticosteroids for severe IPF exacerbations are warranted, though our finding that corticosteroids are near-ubiquitous highlights the difficulty in conducting such studies given perceived lack of equipoise.
Older studies have illustrated much higher rates of immunosuppressive therapy (over 60)%,29 and our findings of infrequent use of immunosuppressive and/or antioxidant therapy with low variation between centres suggests that deadoption has occurred in recent years given poor evidence supporting any of these medications in IPF.4 8 We were unable to assess the use of antifibrotics agents (nintendanib and pirfenidone) given the inaccuracy of charge codes for specialty pharmacy medications; these have not demonstrated benefit in acute exacerbations to date.8 30
Our risk-adjusted median hospital mortality rate of 16% (11%–24%) (32% when including discharges to hospice), with relatively little between-hospital variation, is comparable to the findings of recent, large, multicentre studies, which found mortality for patients with IPF admitted to the ICU around 20%–30%.4 Notably, these findings demonstrate improved mortality rates for patients with severe IPF exacerbations as compared with prior, single-centre studies, which demonstrated ICU mortality rates ranging from 45% to 80%.31 It is possible that improved mortality reflects overall improvements in ICU care and a better understanding and adoption of lung protective ventilatory strategies over the last two decades.32–35
Our study has several strengths. The use of a large, claims-based database allowed us to assess outcomes for large numbers of patients admitted to a wide range of hospitals and likely reflect nationwide outcomes more accurately than single-centre studies. We used a modified version of a validated ICD-10-based algorithm to identify patients with IPF,2 which excluded other ILDs and autoimmune conditions that could cause an IPF-like ILD. We also included hospital-level covariates that have not been included in prior studies, including lung-transplant centre status and safety net hospital status in order to account for hospital characteristics that may affect outcomes. Further, our sensitivity analysis restricting to only patients with IPF as primary diagnoses yielded similar results.
Our study also has limitations. First, outpatient records were not available in order to ascertain method of IPF diagnosis, baseline severity of IPF, outpatient steroid or immunosuppressive use, or posthospitalisation outcomes. We also could not capture whether hospitals were ILD centres, which are difficult to define. Second, due to the nature of the administrative database, we could not assess whether included patients met diagnostic criteria for an acute exacerbation of IPF as defined in the 2016 American Thoracic Society International Working Group report.8 ICD and charge codes may misclassify patients included in the study, covariates, and the practices measured as outcomes. However, as in the prior literature, we limited our inclusion criteria to patients with either a primary diagnosis of IPF2 3 or with a primary diagnosis of acute respiratory failure and a secondary diagnosis of IPF in order to maximise the chance that patients were admitted for their IPF; our sensitivity analysis of patients with IPF as a primary or admitting diagnosis yielded similar results. Other ICD and charge code algorithms, including adjustment for severity of acute illness, have also been previously validated.15–17 Third, there may be unmeasured drivers of variation in IMV; further studies are needed to assess the factors affecting decisions to pursue IMV. Finally, the high rate of corticosteroid use suggests further study is warranted assessing both effectiveness as well as variability in route, timing and duration of corticosteroid use (not assessed here) in order to understand optimal corticosteroid practices.
Conclusion
In a large, multicentre study of patients with severe IPF exacerbations, we observed high variation in the use of IMV and NIMV and lower variation in corticosteroid use and immunosuppressant and/or antioxidant use among patients hospitalised with severe IPF exacerbations. Further research is needed to guide the decision to initiate IMV and use NIMV and to understand the effectiveness of corticosteroids among patients with severe IPF exacerbations.
Footnotes
Contributors: DAS, AJW and ACL were involved in the planning of this study. DAS, AJW, FJH, NAB, DP and ACL were involved in the data collection and data analysis of this study. DAS, AJW, FJH, NAB and ACL were involved in writing the manuscript for this study. DAS submitted the study. DAS, AJW, and ACL are the guarantors of the study.
Funding: DAS: NIH T32 HL007035. AJW: NIH R01HL139751, NIH R01HL151607, NIH R01HL136660 and NIH OT2HL156812-01. FJH: Cystic Fibrosis Foundation: Regenerating Airway Epithelium with Basal Cells Derived from Human iPSCs, Cystic Fibrosis Foundation: De-Novo Generation of Pulmonary ionocytes from Human Pluripotent Stem Cells, NIH 5R01HL139799-04, NIH 5U01HL148692-02. NAB: NIH NCATS 1KL2TR001411. ACL: NIH K23HL 153482, Boston University School of Medicine Department of Medicine Career Investment Award, Doris Duke Charitable Foundation Fund to Retain Clinician Scientists
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as online supplemental information. ICD and Charge codes used to obtain data are included in the online supplemental material. The full dataset is available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study was designated not Human Subjects Research by Boston University’s Institutional Review Board (#H-41991) and was exempt from ethical approval.
References
- 1.Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 2018;198:e44–68. 10.1164/rccm.201807-1255ST [DOI] [PubMed] [Google Scholar]
- 2.Durheim MT, Judy J, Bender S, et al. In-hospital mortality in patients with idiopathic pulmonary fibrosis: a US cohort study. Lung 2019;197:699–707. 10.1007/s00408-019-00270-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Durheim MT, Judy J, Bender S, et al. A retrospective study of in-hospital mortality in patients with idiopathic pulmonary fibrosis between 2015 and 2018. Medicine (Baltimore) 2020;99:e23143. 10.1097/MD.0000000000023143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schrader M, Sathananthan M, Jeganathan N. Patients with idiopathic pulmonary fibrosis admitted to the ICU with acute respiratory failure-a reevaluation of the risk factors and outcomes. J Intensive Care Med 2022;37:342–51. 10.1177/0885066621989244 [DOI] [PubMed] [Google Scholar]
- 5.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788–824. 10.1164/rccm.2009-040GL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rush B, Wiskar K, Berger L, et al. The use of mechanical ventilation in patients with idiopathic pulmonary fibrosis in the United States: a nationwide retrospective cohort analysis. Respir Med 2016;111:72–6. 10.1016/j.rmed.2015.12.005 [DOI] [PubMed] [Google Scholar]
- 7.Raghu G, Remy-Jardin M, Richeldi L, et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 2022;205:e18–47. 10.1164/rccm.202202-0399ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collard HR, Ryerson CJ, Corte TJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis. an international working group report. Am J Respir Crit Care Med 2016;194:265–75. 10.1164/rccm.201604-0801CI [DOI] [PubMed] [Google Scholar]
- 9.Naccache J-M, Montil M, Cadranel J, et al. Study protocol: exploring the efficacy of cyclophosphamide added to corticosteroids for treating acute exacerbation of idiopathic pulmonary fibrosis; a randomized double-blind, placebo-controlled, multi-center phase III trial (EXAFIP). BMC Pulm Med 2019;19:75. 10.1186/s12890-019-0830-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horita N, Akahane M, Okada Y, et al. Tacrolimus and steroid treatment for acute exacerbation of idiopathic pulmonary fibrosis. Intern Med 2011;50:189–95. 10.2169/internalmedicine.50.4327 [DOI] [PubMed] [Google Scholar]
- 11.Donahoe M, Valentine VG, Chien N, et al. Correction: autoantibody-targeted treatments for acute exacerbations of idiopathic pulmonary fibrosis. PLoS One 2015;10:e0133684. 10.1371/journal.pone.0133684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thabut G, Mal H, Castier Y, et al. Survival benefit of lung transplantation for patients with idiopathic pulmonary fibrosis. J Thorac Cardiovasc Surg 2003;126:469–75. 10.1016/s0022-5223(03)00600-7 [DOI] [PubMed] [Google Scholar]
- 13.Yusen RD, Edwards LB, Kucheryavaya AY, et al. The registry of the international society for heart and lung transplantation: thirty-second official adult lung and heart-lung transplantation report--2015; focus theme: early graft failure. J Heart Lung Transplant 2015;34:1264–77. 10.1016/j.healun.2015.08.014 [DOI] [PubMed] [Google Scholar]
- 14.White Pap Prem Hosp Database . Premier healthcare database: data that informs and performs; 2022. Available: https://products.premierinc.com/downloads/PremierHealthcareDatabaseWhitepaper.pdf [Accessed 28 Feb 2022].
- 15.Sun JW, Rogers JR, Her Q, et al. Adaptation and validation of the combined comorbidity score for ICD-10-CM. Med Care 2017;55:1046–51. 10.1097/MLR.0000000000000824 [DOI] [PubMed] [Google Scholar]
- 16.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001;29:1303–10. 10.1097/00003246-200107000-00002 [DOI] [PubMed] [Google Scholar]
- 17.Bosch NA, Law AC, Rucci JM, et al. Predictive validity of the sequential organ failure assessment score versus claims-based scores among critically ill patients. Ann Am Thorac Soc 2022;19:1072–6. 10.1513/AnnalsATS.202111-1251RL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hefner JL, Hogan TH, Opoku-Agyeman W, et al. Defining safety net hospitals in the health services research literature: a systematic review and critical appraisal. BMC Health Serv Res 2021;21:278. 10.1186/s12913-021-06292-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sutton JP, Fingar KR. Characteristics of safety-net hospitals; 2014. 19. [PubMed]
- 20.Lasser KE, Liu Z, Lin M-Y, et al. Changes in hospitalizations at US safety-net hospitals following medicaid expansion. JAMA Netw Open 2021;4:e2114343. 10.1001/jamanetworkopen.2021.14343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merlo J, Chaix B, Ohlsson H, et al. A brief conceptual tutorial of multilevel analysis in social epidemiology: using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Community Health 2006;60:290–7. 10.1136/jech.2004.029454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seymour CW, Iwashyna TJ, Ehlenbach WJ, et al. Hospital-level variation in the use of intensive care. Health Serv Res 2012;47:2060–80. 10.1111/j.1475-6773.2012.01402.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Merlo J, Yang M, Chaix B, et al. A brief conceptual tutorial on multilevel analysis in social epidemiology: investigating contextual phenomena in different groups of people. J Epidemiol Community Health 2005;59:729–36. 10.1136/jech.2004.023929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mooney JJ, Raimundo K, Chang E, et al. Mechanical ventilation in idiopathic pulmonary fibrosis: a nationwide analysis of ventilator use, outcomes, and resource burden. BMC Pulm Med 2017;17:84. 10.1186/s12890-017-0426-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marchioni A, Tonelli R, Rossi G, et al. Ventilatory support and mechanical properties of the fibrotic lung acting as a “squishy ball.” Ann Intensive Care 2020;10:13. 10.1186/s13613-020-0632-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhatti H, Girdhar A, Usman F, et al. Approach to acute exacerbation of idiopathic pulmonary fibrosis. Ann Thorac Med 2013;8:71–7. 10.4103/1817-1737.109815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brereton CJ, Jo HE. Acute exacerbations of idiopathic pulmonary fibrosis and the role of corticosteroids. Breathe (Sheff) 2020;16:200086. 10.1183/20734735.0086-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farrand E, Vittinghoff E, Ley B, et al. Corticosteroid use is not associated with improved outcomes in acute exacerbation of IPF. Respirology 2020;25:629–35. 10.1111/resp.13753 [DOI] [PubMed] [Google Scholar]
- 29.Song JW, Hong S-B, Lim C-M, et al. Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. Eur Respir J 2011;37:356–63. 10.1183/09031936.00159709 [DOI] [PubMed] [Google Scholar]
- 30.Petnak T, Lertjitbanjong P, Thongprayoon C, et al. Impact of antifibrotic therapy on mortality and acute exacerbation in idiopathic pulmonary fibrosis: a systematic review and meta-analysis. Chest 2021;160:1751–63. 10.1016/j.chest.2021.06.049 [DOI] [PubMed] [Google Scholar]
- 31.Huapaya JA, Wilfong EM, Harden CT, et al. Risk factors for mortality and mortality rates in interstitial lung disease patients in the intensive care unit. Eur Respir Rev 2018;27:180061. 10.1183/16000617.0061-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zimmerman JE, Kramer AA, Knaus WA. Changes in hospital mortality for United States intensive care unit admissions from 1988 to 2012. Crit Care 2013;17:R81. 10.1186/cc12695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cochi SE, Kempker JA, Annangi S, et al. Mortality trends of acute respiratory distress syndrome in the United States from 1999 to 2013. Ann Am Thorac Soc 2016;13:1742–51. 10.1513/AnnalsATS.201512-841OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rittayamai N, Brochard L. Recent advances in mechanical ventilation in patients with acute respiratory distress syndrome. Eur Respir Rev 2015;24:132–40. 10.1183/09059180.00012414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kallet RH, Jasmer RM, Pittet J-F, et al. Clinical implementation of the ARDS network protocol is associated with reduced hospital mortality compared with historical controls. Crit Care Med 2005;33:925–9. 10.1097/01.ccm.0000162382.59289.9c [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2022-001593supp001.pdf (214.8KB, pdf)
Data Availability Statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as online supplemental information. ICD and Charge codes used to obtain data are included in the online supplemental material. The full dataset is available upon reasonable request.