Figure 2.
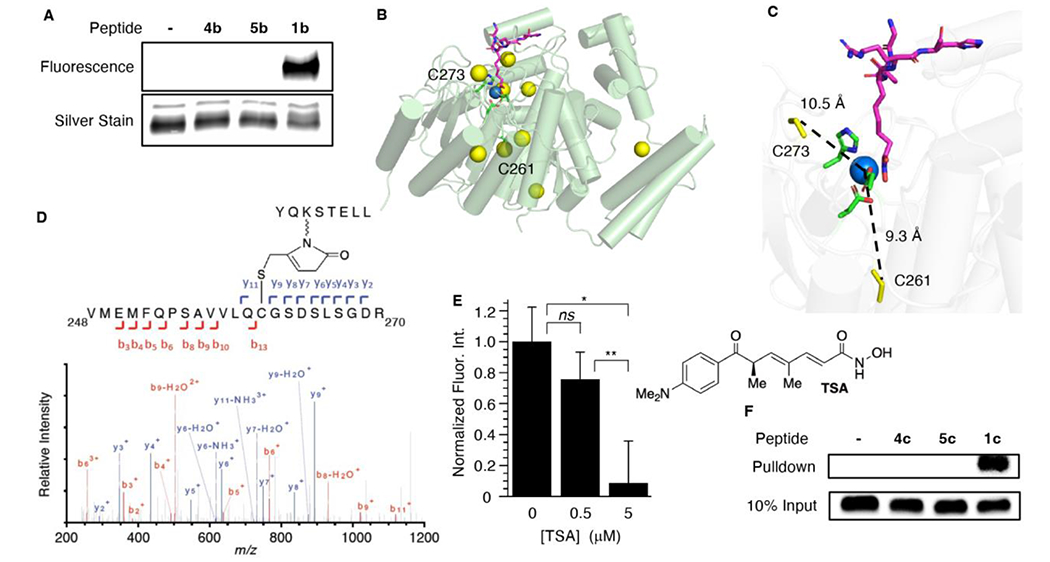
Covalent modification of HDAC1 by a KMP-containing peptide (H3K56MP). A) Covalent modification of HDAC1 (0.1 μM) by fluorescently labeled KMP-containing histone peptide (1b, 50 μM). B) X-ray crystal structure of HDAC1 bound with a peptide inhibitor (PDB: 5ICN), showing active site zinc (blue) and 9 cysteine residues (yellow). C) Active site showing the distance between Zn2+ and C261, C273. D) LC-MS/MS analysis of HDAC1 trypsin fragment V248-R270 that is covalently modified by a histone peptide containing H3K56MP (1a). E) Trichostatin A (TSA) competition for covalent modification of HDAC1 (100 nM) by KMP-containing histone peptide H3K56MP (1b, 5 μM). Average fluorescence intensity of reaction in the absence of TSA is normalized to 1.0. Data are presented as the ave. ± std. dev. (n=3). (ns) indicates not significantly different, (*) indicates p < 0.0005 and (**) indicates p < 0.005. F) Covalent modification of HDAC1 by histone peptide containing H3K56MP (1c, 10 μM) in HeLa cell nuclear lysate. Detection is carried out using anti-HDAC1 antibody. HDAC1 migrates at ~60 kDa on the gel (Figure S1).
