Fig. 1.
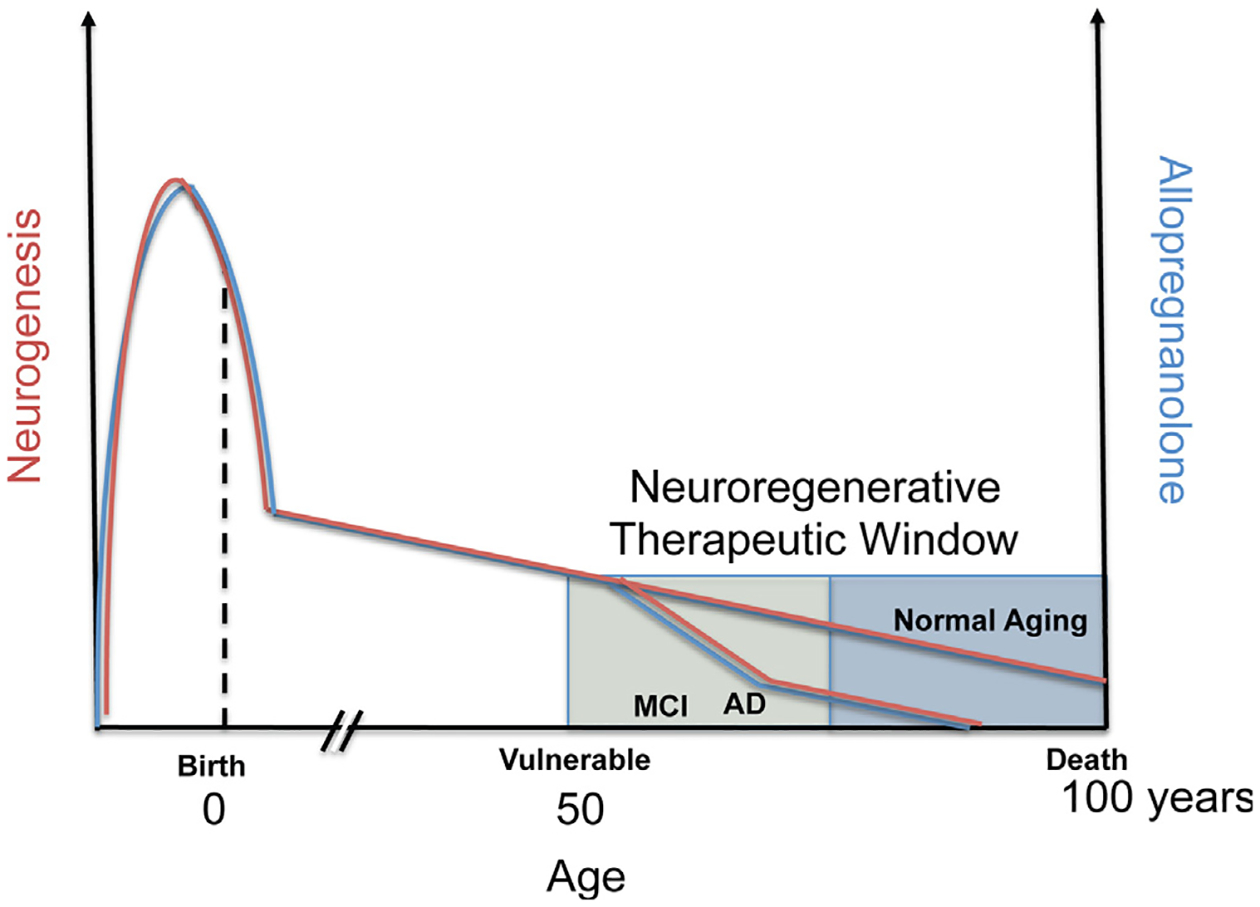
Optimal allopregnanolone (Allo) therapeutic regimen applied to therapeutic window to protect from Alzheimer’s disease (AD). Endogenous Allo (blue colored line) is produced both locally within the brain and from peripheral sources, including maternal circulation pre-natal, and post-natal and adult from the gonads and adrenal cortex in both male and female. In this generalized diagram, Allo and neurogenesis (red colored line) correlate throughout the life span and in disease. Most notable is the elevated level of Allo during fetal brain development that peaks and then sharply declines before birth (Hirst et al., 2009; Nguyen et al., 2003; Westcott et al., 2008; Luisi et al., 2000). Not depicted are the cyclic fluctuations in progesterone and closely follows progesterone levels in vivo. Also not depicted are the fluctuations in Allo during puberty. In adults, levels of neurosteroids including Allo gradually decline with advanced age and can be associated with chronological age or more specifically by what is often called endocrine age which considers the unique or categorical endocrine system status. In the presymptomatic and mild cognitive impairment (MCI) stages before AD, circulating blood and brain cortex Allo levels sharply decline correlating with onset of AD (Marx et al., 2006; Naylor et al., 2010). In most animal models of AD, the decline in neurogenesis correlates with temporal progression of AD pathology (Lazarov and Marr, 2010). Human neurogenesis declines with age and doublecortin-positive cells decreased approximately ten-fold from 0 to 100 years of age (Knoth et al., 2010) and human data roughly parallel the rate of neurogenesis in aging rodent models. Another method in which post-mortem brain genomic DNA samples containing unique radiometric signatures due to a spike in atmospheric 14C levels from global nuclear bomb fallout were used to determine neuronal and nonneuronal cell birthdates (Spalding et al., 2013). The results of these studies revealed that the age of the 14C-labeled DNA within adult hippocampal neurons were incorporated during adulthood. Each year approximately 1.75 percent of neurons turned over within the self-renewing fraction with only a modest decline during aging. A best-fit scenario model predicted that approximately 35 percent of the hippocampal cells were cycling corresponding to slightly less than the proportion that constitute the entire dentate gyrus region. Spalding and colleagues estimated that the hippocampal dentate gyrus of human brain produces around 700 new neurons per day. At this rate, enough neurons could be regenerated in the hippocampus to replace the dentate gyrus region over the human lifespan suggesting that the potential amount of neurogenesis is very substantial. Interestingly, the rate of neurogenesis in adult humans was similar by comparison to the rate determined in adult rodents but the rate did not decline as steeply as is known in rodents suggesting that humans may rely on neurogenesis more during the aging process. Chronic stress is associated with depressed responsiveness to constant levels of Allo and in correlation, chronic stress inhibits neurogenesis and exacerbates AD and loss of memory (not depicted) (Bengtsson et al., 2012, 2012). Further, neurodegeneration, a hallmark of AD negatively correlates with levels of Allo (also not depicted). The graphical representation suggests a prominent role of Allo in the neuroendocrine axis, its role both in neurogenesis and AD progression.
