Abstract
Cutaneous leishmaniasis (CL) is endemic to Israel. Previously, CL caused by Leishmania infantum had been reported in Israel only once (in 2016). We report 8 L. infantum CL cases; 7 occurred during 2020–2021. None of the patients had systemic disease. L. infantum CL may be an emerging infection in Israel.
Keywords: cutaneous leishmaniasis, Leishmania infantum, parasites, zoonoses, emerging infection, Israel
Leishmania is a protozoan that causes a wide variety of diseases. Clinical manifestations include cutaneous leishmaniasis (CL), visceral leishmaniasis (VL), or mucocutaneous leishmaniasis, depending mainly on the specific causative leishmania species (1). Old World CL is classically attributable to L. major and L. tropica. However, L. infantum, which usually causes VL, recently has been considered as a causative agent of CL (2).
CL is endemic to Israel and has been attributed almost exclusively to infection with L. major and, more recently, to L. tropica (3). However, human VL caused by an L. donovani substrain, L. infantum, has been reported, albeit rarely, in Israel (4). The reservoir is dogs, and the epidemiology of the disease among dogs indicates wide distribution in Israel (5). Nevertheless, cases of CL caused by L. infantum were previously reported in the Middle East and worldwide. In Israel, CL caused by L. infantum was reported in 2016 (6); that infection was acquired in the southern part of Israel, known to be endemic for L. major. In this article, we report 8 cases of L. infantum CL observed in Israel during 2018–2021.
The Study
All patients were seen at Chaim Sheba Medical Center (Tel Hashomer, Israel). We defined L. infantum CL as cutaneous lesions (ulcers, nodules, or papules) clinically compatible with leishmaniasis and a PCR result positive for L. infantum. The study was approved by the Chaim Sheba Medical Center Institutional Review Board (protocol approval no. 7274–09).
We took tissue specimens from suspected skin lesions and extracted DNA from dried blood spots by using the QIAamp DNA Mini Kit (QIAGEN, https://www.qiagen.com). We analyzed the samples for internal transcribed spacer 1 PCR by using 10 μM primers (LITSR 5′-CTGGATCATTTTCCGATG-3′ and L5.8S 5′-TGATACCACTTATCGCACTT-3′) (7). We analyzed the amplicons on 4% agarose gel. We sent the L. infantum–positive samples to Hylabs (Rehovot, Israel) for a second validation and sequencing.
During 2016–2021, our laboratory at Chaim Sheba Medical Center diagnosed 609 cases of leishmania (Table 1). Among those, 8 cases (1.3%) were attributable to L. infantum. All of the cases were confirmed by PCR and further sequencing.
Table 1. Leishmaniasis cases diagnosed (N = 609), by Leishmania species, at the laboratory at Chaim Sheba Medical Center, Tel Hashomer, Israel, 2016–2021*.
| Leishmania species | No. (%) cases |
|---|---|
| L. major | 403 (66.1) |
| L. tropica | 175 (28.7) |
| L. braziliensis | 23 (3.7) |
| L. infantum | 8 (1.3) |
One case was diagnosed in 2018 and the other 7 cases during 2020–2021. Of the 8 patients, 6 were male and 2 female. The infections were acquired in different regions of Israel, including the southern region (Negev and Arava areas), where L. major is endemic, and the central region (east of Tel Aviv), where L. tropica is endemic (Figure 1).
Figure 1.
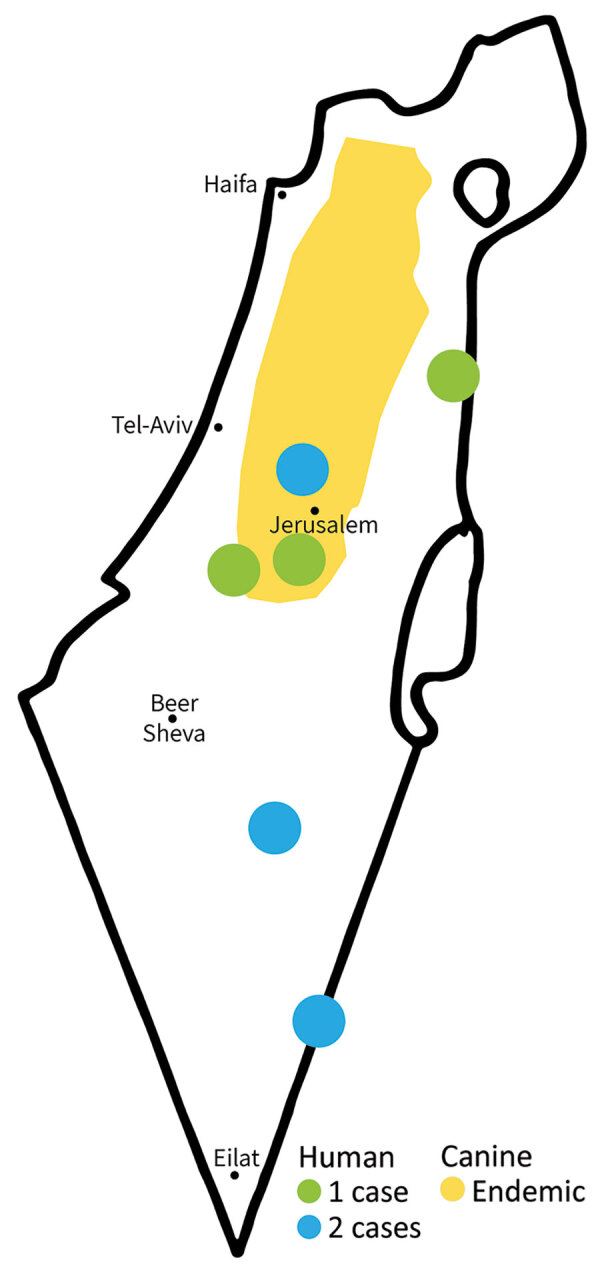
Foci of cutaneous leishmaniasis caused by Leishmania infantum, Israel, 2018–2021. Distribution of canine L. infantum in Israel based on Jaffe et al (8).
The mean age of patients at diagnosis was 31 years (range 10–56 years; median 28.5 years). The average number of lesions was 2 (range 1–6). The lesions were commonly located on the limbs (6 patients); in 2 patients, the lesions were located on the face (Table 2; Figure 2).
Table 2. Clinical and demographic characteristics of 8 patients with cutaneous leishmaniasis caused by Leishmania infantum, Israel, 2018–2021 .
| Characteristic | Value |
|---|---|
| Mean age, y (range) |
31.8 (10–56) |
| Sex, no. patients | |
| M | 6 |
| F |
2 |
| Exposure area |
Southern and eastern Israel |
| Mean no. lesions (range) |
2 (1–6) |
| Anatomic location, no. patients* |
|
| Head and neck | 2 |
| Upper limbs | 3 |
| Lower limbs | 4 |
*Includes multiple locations per patient.
Figure 2.
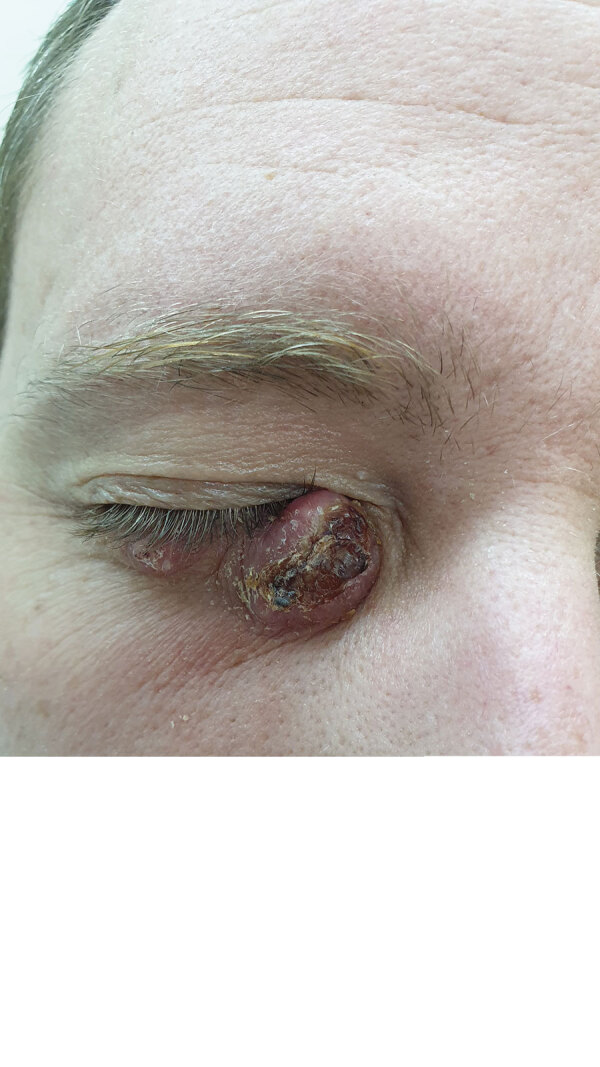
Cutaneous lesion caused by Leishmania infantum on the right lower eyelid of a patient seen at Chaim Sheba Medical Center, Israel, 2021.
Two patients recovered within a short period (≈3 months) without any treatment, 3 patients were treated with topical therapy (intralesional sodium stibogluconate and liquid nitrogen), and the other 3 patients were treated with systemic therapy (oral miltefosine and intravenous liposomal amphotericin B). Systemic therapy was initiated when topical treatment failed or intralesional sodium stibogluconate injection was not feasible because of the anatomic location of the lesions (e.g., on the face or eyelid). All patients who received systemic therapy had a good response. None of the patients had systemic disease.
Conclusions
The first known case of CL attributable to L. infantum infection in southern Israel was reported in 2016 (6). We describe a case series of 8 patients with L. infantum CL in Israel. The disease was acquired in different parts of Israel, and all but 1 case occurred during 2020–2021, pointing to a possible emergence of a new species causing CL in Israel. Canids, including domestic dogs and wild canids, are reservoirs for L. infantum. Recently, cats were also reported as a reservoir (5).
The known vectors of L. infantum are Phlebotomus syriacus, P. tobbi, and P. perfilliewi flies, all of which exist in Israel (9) Because both the reservoir and the vector for L. infantum have existed in Israel for years, the reason for this outbreak of CL is not well understood. Of note, in several countries, including Saudi Arabia, Turkey, and Yemen, L. infantum as a causative agent for CL has already been described (10–12), whereas Israel historically has not documented this disease.
Diagnosis of this Leishmania species in skin lesions can be made mainly by PCR testing. In Israel, PCR tests have been available since 2003 (7). At Chaim Sheba Medical Center, molecular diagnosis has been available since 2016, and it was only recently that the emergence of L. infantum CL was observed, thus excluding a diagnostic bias as a cause for this phenomenon. In addition, a query of 3 other centers in Israel where molecular diagnosis is performed indicated that cases of L. infantum caused by CL were seen only as of 2020 (E. Schwartz, pers. comm., email, 2022 Nov 1).
Clinical pleomorphism is a major feature of Leishmania parasites, a phenomenon particularly well illustrated in the case of L. infantum. This species is endemic in countries around the Mediterranean Basin and is most commonly known to cause VL, which is fatal if untreated. Host factors play an important role in this pleomorphism (13). However, some reports suggest a possible contribution of parasite factors attributable to different subspecies. Among the different L. infantum zymodemes (groups of strains showing the same isoenzymatic profile), some are restricted to cutaneous cases, such as MON-1, MON-24, MON-29, and MON-33 (14). However, the major and ubiquitous MON-1 zymodeme, mostly associated with VL, was also found in CL cases (14).
Transmission of L. infantum infection is considered predominantly zoonotic, and domestic animals are the major reservoir. The disease spreads through expansion of the zoonotic or the anthroponotic cycles (15) to regions where local vectors (phlebotomine species) can contribute to L. infantum transmission.
No specific recommendations exist regarding treatment of CL caused by L. infantum. The treatment regimen we chose was based on disease severity. In mild cases, no treatment was given, and the lesions healed spontaneously and rapidly within 3 months. In moderate cases, topical treatment was used (intralesional sodium stibogluconate and liquid nitrogen). In severe cases or in cases that were refractory to local treatment, systemic treatment was given (oral miltefosine and intravenous liposomal amphotericin B), all with good responses. None of the patients had systemic involvement. The treatment protocols for severe lesions were similar to those used in L. donovani CL cases and in severe cases of L. major and L. tropica CL. Although L. infantum can cause VL, none of the patients had systemic disease. Therefore, a nonsystemic treatment (topical or intralesional) seems to be an adequate treatment in most cases.
In conclusion, the increasing number of L. infantum CL cases since 2020, occurring in different parts of Israel, point to an emerging new leishmania species in Israel. Clinicians should include this pathogen in the differential diagnosis of patients with cutaneous lesions clinically compatible with leishmaniasis.
Biography
Dr. Solomon is dermatologist at Chaim Sheba Medical Center in Israel. Her primary research interests include cutaneous leishmaniasis and tropical skin diseases.
Footnotes
Suggested citation for this article: Solomon M, Astman N, Warshavsky K, Barzilai A, Meningher T, Avni D, et al. Cutaneous leishmaniasis caused by Leishmania infantum, Israel, 2018–2021. Emerg Infect Dis. 2023 May [date cited]. https://doi.org/10.3201/eid2905.221812
References
- 1.Reithinger R, Dujardin JC, Louzir H, Pirmez C, Alexander B, Brooker S. Cutaneous leishmaniasis. Lancet Infect Dis. 2007;7:581–96. 10.1016/S1473-3099(07)70209-8 [DOI] [PubMed] [Google Scholar]
- 2.del Giudice P, Marty P, Lacour JP, Perrin C, Pratlong F, Haas H, et al. Cutaneous leishmaniasis due to Leishmania infantum. Case reports and literature review. Arch Dermatol. 1998;134:193–8. 10.1001/archderm.134.2.193 [DOI] [PubMed] [Google Scholar]
- 3.Solomon M, Pavlotsky F, Leshem E, Ephros M, Trau H, Schwartz E. Liposomal amphotericin B treatment of cutaneous leishmaniasis due to Leishmania tropica. J Eur Acad Dermatol Venereol. 2011;25:973–7. 10.1111/j.1468-3083.2010.03908.x [DOI] [PubMed] [Google Scholar]
- 4.Nasereddin A, Baneth G, Schönian G, Kanaan M, Jaffe CL. Molecular fingerprinting of Leishmania infantum strains following an outbreak of visceral leishmaniasis in central Israel. J Clin Microbiol. 2005;43:6054–9. 10.1128/JCM.43.12.6054-6059.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baneth G, Nachum-Biala Y, Zuberi A, Zipori-Barki N, Orshan L, Kleinerman G, et al. Leishmania infection in cats and dogs housed together in an animal shelter reveals a higher parasite load in infected dogs despite a greater seroprevalence among cats. Parasit Vectors. 2020;13:115. 10.1186/s13071-020-3989-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ben-Shimol S, Sagi O, Horev A, Avni YS, Ziv M, Riesenberg K. Cutaneous leishmaniasis caused by Leishmania infantum in Southern Israel. Acta Parasitol. 2016;61:855–8. 10.1515/ap-2016-0118 [DOI] [PubMed] [Google Scholar]
- 7.Schönian G, Nasereddin A, Dinse N, Schweynoch C, Schallig HD, Presber W, et al. PCR diagnosis and characterization of Leishmania in local and imported clinical samples. Diagn Microbiol Infect Dis. 2003;47:349–58. 10.1016/S0732-8893(03)00093-2 [DOI] [PubMed] [Google Scholar]
- 8.Jaffe CL, Baneth G, Abdeen ZA, Schlein Y, Warburg A. Leishmaniasis in Israel and the Palestinian Authority. Trends Parasitol. 2004;20:328–32. 10.1016/j.pt.2004.05.001 [DOI] [PubMed] [Google Scholar]
- 9.Orshan L, Szekely D, Khalfa Z, Bitton S. Distribution and seasonality of Phlebotomus sand flies in cutaneous leishmaniasis foci, Judean Desert, Israel. J Med Entomol. 2010;47:319–28. 10.1093/jmedent/47.3.319 [DOI] [PubMed] [Google Scholar]
- 10.Eroglu F, Koltas IS, Alabaz D, Uzun S, Karakas M. Clinical manifestations and genetic variation of Leishmania infantum and Leishmania tropica in Southern Turkey. Exp Parasitol. 2015;154:67–74. 10.1016/j.exppara.2015.04.014 [DOI] [PubMed] [Google Scholar]
- 11.Khatri ML, Di Muccio T, Gramiccia M. Cutaneous leishmaniasis in North-Western Yemen: a clinicoepidemiologic study and Leishmania species identification by polymerase chain reaction-restriction fragment length polymorphism analysis. J Am Acad Dermatol. 2009;61:e15–21. 10.1016/j.jaad.2009.04.047 [DOI] [PubMed] [Google Scholar]
- 12.Rasheed Z, Ahmed AA, Salem T, Al-Dhubaibi MS, Al Robaee AA, Alzolibani AA. Prevalence of Leishmania species among patients with cutaneous leishmaniasis in Qassim province of Saudi Arabia. BMC Public Health. 2019;19:384. 10.1186/s12889-019-6710-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pratlong F, Dedet JP, Marty P, Portús M, Deniau M, Dereure J, et al. Leishmania-human immunodeficiency virus coinfection in the Mediterranean basin: isoenzymatic characterization of 100 isolates of the Leishmania infantum complex. J Infect Dis. 1995;172:323–6. 10.1093/infdis/172.1.323 [DOI] [PubMed] [Google Scholar]
- 14.Rioux JA, Moreno G, Lanotte G, Pratlong F, Dereure J, Rispail P. Two episodes of cutaneous leishmaniasis in man caused by different zymodemes of Leishmania infantum s.l. Trans R Soc Trop Med Hyg. 1986;80:1004–5. 10.1016/0035-9203(86)90300-7 [DOI] [PubMed] [Google Scholar]
- 15.Svobodová M, Alten B, Zídková L, Dvorák V, Hlavacková J, Mysková J, et al. Cutaneous leishmaniasis caused by Leishmania infantum transmitted by Phlebotomus tobbi. Int J Parasitol. 2009;39:251–6. 10.1016/j.ijpara.2008.06.016 [DOI] [PubMed] [Google Scholar]


