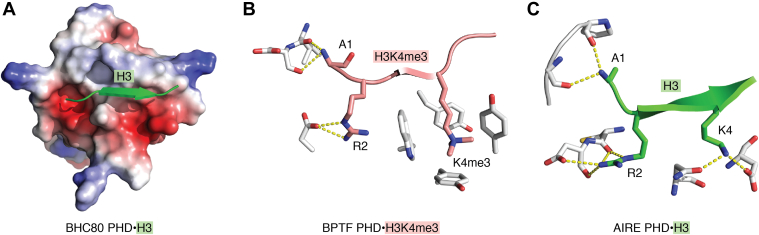
Figure 1.
Canonical histone H3-binding mechanism of PHD fingers.A, the crystal structure of the BHC80 PHD finger in complex with the unmodified histone H3 peptide (PDB ID 2PUY). Electrostatic surface potential of the PHD finger is shown with blue and red colors representing surface positive and negative charges, respectively. The H3 peptide is depicted as green ribbon. B, a ribbon diagram of the H3K4me3-binding site derived from the crystal structure of the BPTF PHD finger (gray) in complex with the H3K4me3 peptide (dark pink) (PDB ID 2F6J). Intermolecular hydrogen bonds are indicated by yellow dash lines. The H3K4me3 peptide residues are shown as sticks and labeled. C, a ribbon diagram of the unmodified H3-binding site derived from the crystal structure of the AIRE PHD finger (gray) in complex with the H3 peptide (green) (PDB ID 2KE1). Intermolecular hydrogen bonds are indicated by yellow dash lines. The H3 peptide residues are shown as sticks and labeled. PHD, plant homeodomain.