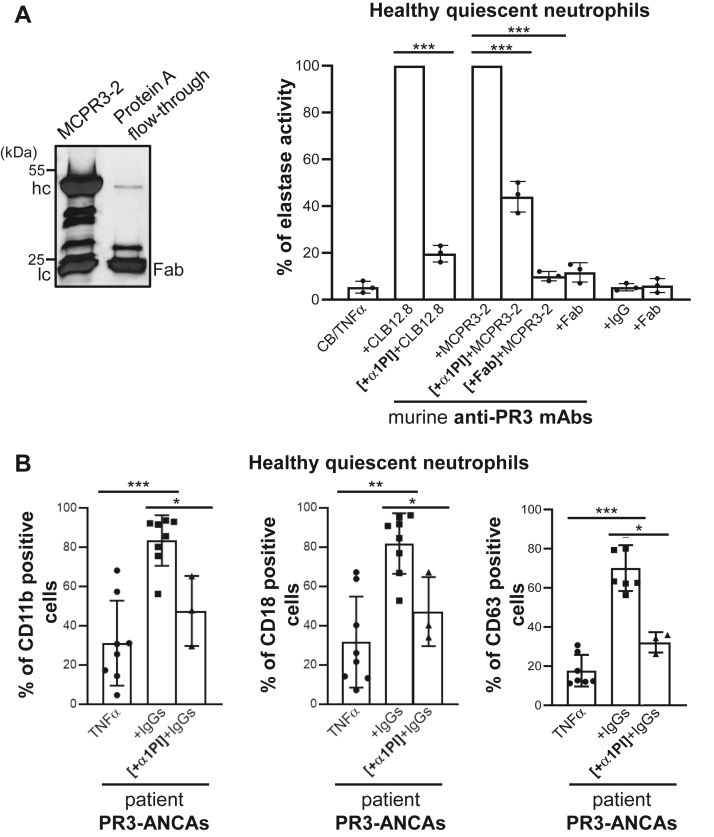
Figure 6.
Effects of α1PI and purified Fab fragments on degranulation by anti-PR3–stimulated neutrophils.A, effects of α1PI and purified Fab fragments on secretion of elastase activity from neutrophils stimulated by anti-PR3 antibodies. (Left) SDS-PAGE (reducing conditions) of Fab fragments prepared from anti-PR3 MCPR3-2 mAbs after digestion with papain. Contaminating intact immunoglobulins and intact Fc parts were removed by the protein A affinity column. IgG heavy and light chains migrate respectively with an apparent molecular weight near 50 and 25 kDa, while Fab fragments display as a single band of 25 kDa. (Right) Purified neutrophils from healthy volunteers were primed, then incubated with anti-PR3 CLB12.8 (40 μg/ml), anti-PR3 WGM-2 (40 μg/ml), or anti-PR3 MCPR3-2 (40 μg/ml) as reported in Figure 5. The residual proteolytic activity of elastase in cell-free neutrophil supernatants was measured as an indicator of cell degranulation induced by anti-PR3 mAbs. Preincubation of the primed-cells with α1PI (50 μM) caused only a partial (50–60%) reduction in anti-PR3 mAbs-induced degranulation. Purified Fab fragments of anti-PR3 CLB12.8 or anti-PR3 MCPR3-2 mAbs were incubated with primed-neutrophils before the cells were treated with the entire antibody in a molar ratio of 4 and the cell degranulation measured as reported above. Results show that Fab fragments compete with the whole antibody for binding to both constitutive and inducible PR3mb, which results in a significant decrease in cell activation. The histogram shows the mean ± SD of three independent experiments, each done in duplicates. Statistical analysis of the data was performed using two-way ANOVA (Bonferroni’s multiple comparison tests), ∗∗∗p < 0.001. B, surface markers were analyzed using TNFα-primed neutrophils stimulated with IgGs from patients (200 μg/ml) before (eight independent experiments for CD11b and CD11b; seven independent experiments for CD63) and after incubation with α1PI (three independent experiments) as in A. After stimulation, cells were washed and stained with CD63-FITC or double stained with CD11b VioBlue/CD18 FITC before flow cytometry analysis. Results show that preincubation of the primed cells with α1PI caused only a partial reduction in anti-PR3–induced degranulation. Histogram shows the mean ± SD of independent experiments. Statistical analysis of the data was performed using a nonparametric test (Mann-Whitney test), ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. α1PI, alpha-1 protease inhibitor; PR3, proteinase 3.