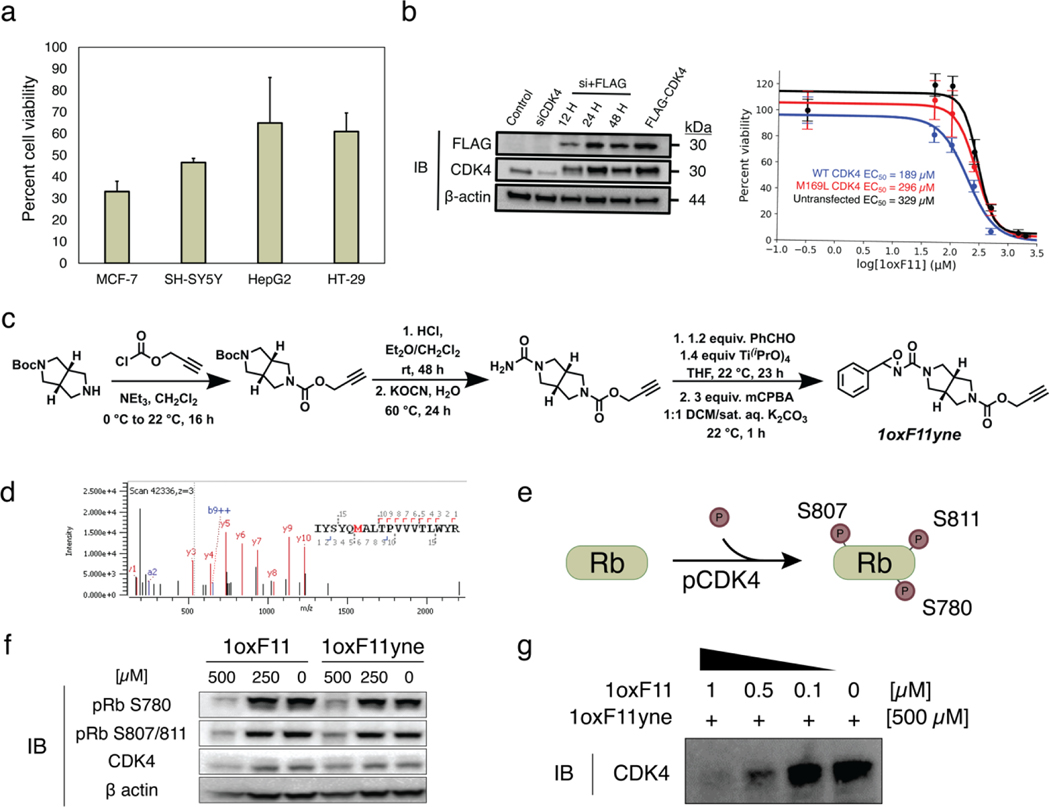
Figure 5.
Decrease in cell viability and inhibition of cellular CDK4 activity by 1oxF11 and its 1oxF11yne analog in various cancer cell models. (a) Screening of ribociclib-sensitive cell lines by treatment with 500 μM 1oxF11 showing differential cell viability profiles across cancer types. The MCF-7 cell line displays the highest sensitivity to 1oxF11. Error bars represent standard deviation of n = 3 biological replicates. (b) Left: Immunoblot timecourse of constitutive knockdown and overexpression of transiently expressed CDK4. Right: Dose-response curves of untransfected and transfected MCF-7 cells transiently expressing CDK4 treated with 1oxF11. Error bars represent standard deviation of n = 3 biological replicates. (c) Synthetic route of 1oxF11yne bearing an alkyne handle for bio-orthogonal detection and enrichment purposes. (d) MS2 data showing that 1oxF11yne is a covalent modifier of CDK4 at the same M169 site as the parent 1oxF11 fragment. (e) Simplified scheme of cellular CDK4 activity, where Rb represents the native substrate of CDK4. Immunoblot analyses measuring the extent of Rb phosphorylation provides a method to detect intracellular CDK4 activity. (f) Immunoblot analysis of MCF-7 cells treated with a titration of 1oxF11 and 1oxF11yne to assess changes in cellular CDK4 activity. (g) Competition binding assay between 1oxF11 and 1oxF11yne, providing evidence for target engagement in MCF-7 cells.