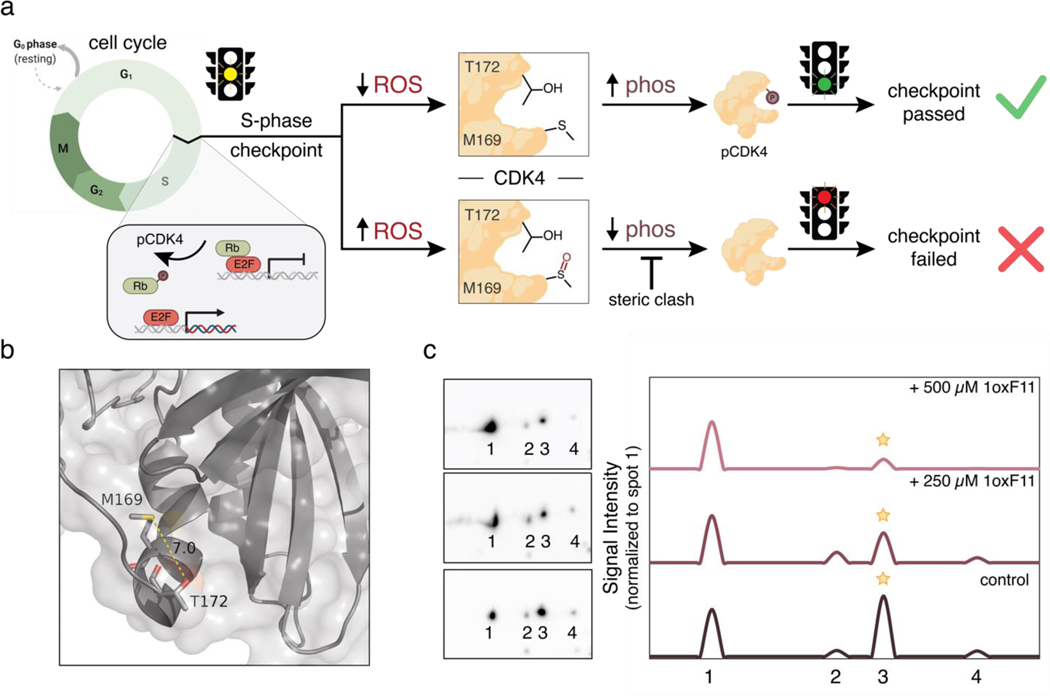
Figure 7.
The ReACT covalent ligand probe platform enables discovery of a reciprocal oxidation/phosphorylation crosstalk pathway in CDK4 through proximal allosteric M169 and T172 sites, where M169-targeted oxidation can inhibit CDK4 activity by preventing phosphorylation at T172. (a) Schematic outlining oxidation/phosphorylation crosstalk between CDK4 M169 and T172. Under low oxidative conditions, T172 of CDK4 is phosphorylated by cyclin-dependent activating kinase (CAK) as part of a critical activating step leading to cell division to pass the S-phase checkpoint. High oxidative conditions can lead to oxidation at M169, which blocks the T172 phosphorylation site, thus preventing cell division via S-phase checkpoint failure. This crosstalk identifies a methionine redox-dependent vulnerability for potential therapeutic intervention. (b) Ribbon structure (PDB: 2W9Z) highlighting proximity of M169 and T172. (c) Monitoring CDK4 phosphorylation status using 2D-immunoblot analyses with phospho-specific CDK4 antibodies. Phosphorylation at T172 decreases with increasing concentrations of added 1oxF11 in MCF-7 cells. Spots 1, 2, 3, and 4 represent different phosphorylation states of CDK4, with spot 1 being unphosphorylated and spot 3 being monophosphorylated at T172. Peaks represent changes in intensity of spots normalized to peak 1 intensity. A clear decrease in spot 3 is observed upon treatment with increasing doses of 1oxF11, consistent with a model where this covalent ligand inhibits CDK4 activity by promoting M169 oxidation to block T172 phosphorylation.