Figure 4. Characterizing the mechanism of the CFTR DUBTAC NJH-2-057.
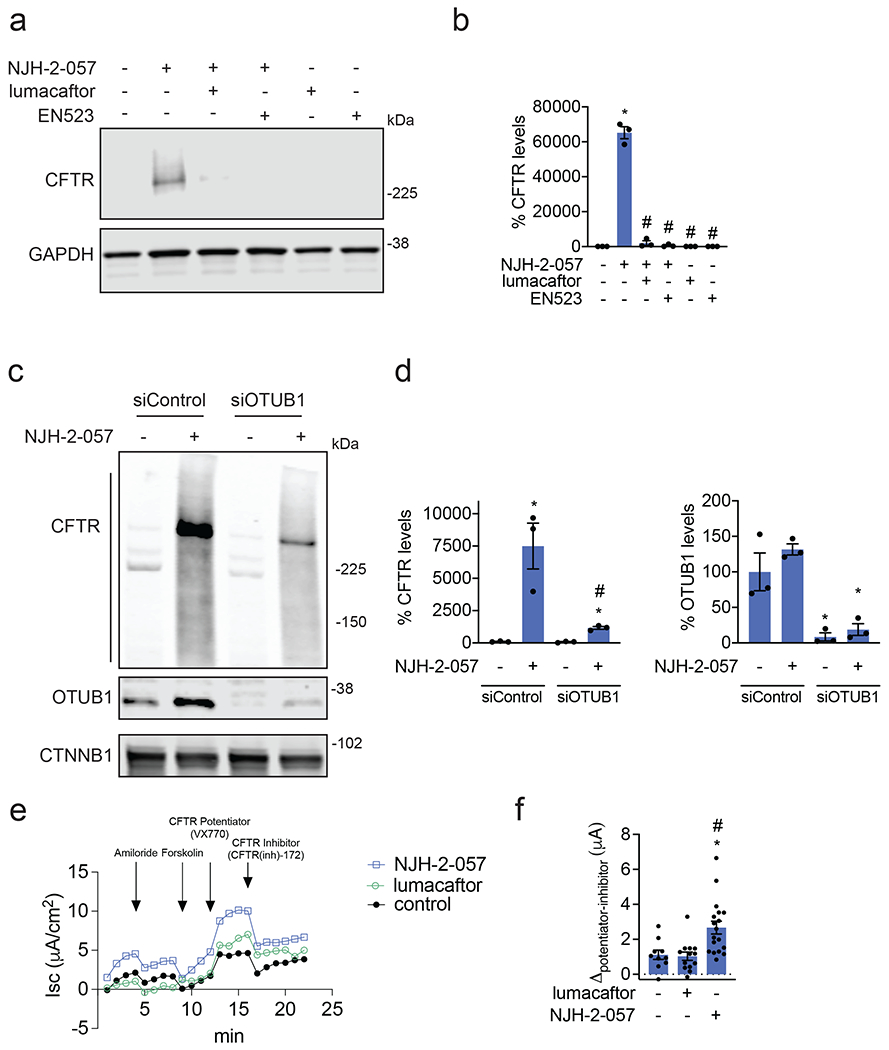
(a) Effect of lumacaftor or EN523 pre-incubation on NJH-2-057 DUBTAC-mediated stabilization of mutant CFTR levels. CFBE41o-4.7 cells expressing ΔF508-CFTR were pre-treated with vehicle DMSO, lumacaftor (100 μM), or EN523 (100 μM) for 1 h prior to treatment with NJH-2-057 (10 μM) for 24 h. Mutant CFTR and loading control GAPDH levels were assessed by Western blotting. (b) Quantification of the experiment described in (a). (c) Effect of OTUB1 knockdown on NJH-2-057 DUBTAC-mediated mutant CFTR stabilization. CFBE41o-4.7 cells expressing ΔF508-CFTR were transiently transfected with siControl or siOTUB1 oligonucleotides for 48 h prior to treatment of cells with vehicle DMSO or NJH-2-057 (10 μM) for 16 h. Mutant CFTR, OTUB1, and loading control GAPDH levels were assessed by Western blotting. (d) Levels of mutant CFTR and OTUB1 from the experiment described in (c). (e) Transepithelial conductance in primary human cystic fibrosis donor bronchial epithelial cells bearing the ΔF508-CFTR mutation. Cells were treated with DMSO vehicle, NJH-2-057 (10 μM), or lumacaftor (10 μM) 24 h prior to the TECC24 assay in which cells received four additional sequential treatments with a sodium channel inhibitor amiloride (10 μM), cAMP activator Forskolin (20 μM), a CFTR potentiator VX770 (0.5 μM), and finally with a CFTR inhibitor CFTR-Inh172 (30 μM). Shown are the average values from conductance from a single donor. Experiments were conducted in primary cells from two donors. (f) Changes in current between potentiator VX770 (Ivacaftor) treatment and the CFTR inhibitor treatment in the experiment described in (e) in two primary human cystic fibrosis donor bronchial epithelial cells bearing the ΔF508-CFTR mutation. Individual replicate data are shown in the bar graph from n=10 biologically independent samples in the DMSO vehicle treated group, n=13 biologically independent samples in the lumacaftor treated group, and n=18 biologically independent samples in the NJH-2-057 treated group. Gels shown in (a, c) are representative of n=3 biologically independent samples/group. Data in (b, d) show individual biological replicate values and average ± sem from n=3 biologically independent samples/group. Statistical significance in was calculated with unpaired two-tailed Student’s t-tests in (b, d, f) and is expressed as *p<0.05 compared to vehicle-treated control in (b, f) and vehicle-treated siControl in (d) and #p<0.05 compared to the NJH-2-057 treated group in (b) and NJH-2-057 treated siControl group for CFTR levels in (d).
