Abstract
Objective
Pancreatic cancer is a globally frequent cause of death, which can be caused by many factors. This meta-analysis was performed to assess the correlation between pancreatic cancer and metabolic syndrome (MetS).
Methods
Publications were identified by searching PubMed, EMBASE, and the Cochrane Library for studies published until November 2022. Case-control and cohort studies published in English that provided information on the odds ratio (OR), relative risk (RR), or hazard ratio (HR) of metabolic syndrome and pancreatic cancer were included in the meta-analysis. Two researchers separately retrieved the core data from the included Random effects meta-analysis was conducted to summarize the findings. Results were presented as relative risk (RR) and 95% confidence interval (CI).
Results
MetS showed a strong association with an increased risk of developing pancreatic cancer (RR1.34, 95% CI1.23–1.46, P<0.001), and gender differences were also observed (men: RR 1.26, 95% CI 1.03–1.54, P=0.022; women: RR 1.64, 95% CI 1.41–1.90, P< 0.001). Moreover, an increased risk of developing pancreatic cancer was strongly linked to hypertension, poor high-density lipoprotein cholesterol, and hyperglycemia (hypertension: RR 1.10 CI 1.01–1.19, P=0.027; low high-density lipoprotein cholesterol: RR 1.24 CI 1.11–1.38, P<0.001; hyperglycemia: RR 1.55, CI 1.42–1.70, P< 0.001). However, pancreatic cancer was independent of obesity and hypertriglyceridemia (obesity: RR 1.13 CI 0.96–1.32, P=0.151, hypertriglyceridemia: RR 0.96, CI 0.87–1.07, P=0.486).
Conclusions
Although further prospective studies are required for confirmation, this meta-analysis indicated a strong relationship between MetS and pancreatic cancer. Regardless of gender, a greater risk of pancreatic cancer existed in people with MetS. Patients with MetS were more likely to develop pancreatic cancer, regardless of gender. Hypertension, hyperglycemia, and low HDL-c levels may largely account for this association. Further, the prevalence of pancreatic cancer was independent of obesity and hypertriglyceridemia.
Systematic review registration
https://www.crd.york.ac.uk/prospero/, identifier CRD42022368980.
Keywords: metabolic syndrome, pancreatic cancer, metabolic component, meta-analysis, pancreas
Introduction
Pancreatic cancer (PC) is a common malignant tumor type with the 12th-highest incidence rate among all malignant tumors (1). PC has a dismal prognosis, with a general five-year relative survival rate of 10%, and it is the fourth and sixth most widely occurring common cause of cancer-related mortality in China and the United States, respectively (2, 3).The risk factors are unclear, and PC may develop in patients with a family history of cancer as well as those who smoke, drink alcohol, are obese, or have diabetes (4).
The metabolic syndrome (MetS) has attracted considerable attention with regard to its association with cardiovascular risk factors, first proposed in 1988 (5).Dyslipidemia, central obesity, poor glucose tolerance, insulin resistance, type 2 diabetes, and hyperinsulinemia are some abnormal metabolic parameters characterizing MetS (6). These parameters are typically assessed using the following indicators: blood pressure, fasting plasma glucose level, waist size, high-density lipoprotein cholesterol (HDL-c) levels, and triglyceride level (7). MetS or its components may be linked to numerous malignancies, including breast, colorectal, endometrial, and gastric cancer (8–11). MetS were also investigated as a potential PC risk factor. It was observed that in the general public, it was strongly linked to an elevated risk of developing PC (12). Previously, the number of MetS components and the probability of developing PC showed a strong correlation (13). The risk of PC varied among people with MetS, with the presence of four or five metabolic components being linked to the highest risk (14). However, a Japanese study found that only women with two or more metabolic components showed an elevated risk of PC (15). A subsequent prospective study, including over 580,000 people, also supported these findings (16). However, several shortcomings of these studies, including insufficient sample size, lack of ethnic/racial heterogeneity, and an inadequate assessment of confounders and/or reverse causality, resulted in contradictory findings.
Several studies have demonstrated that various aspects of MetS, such as obesity and type 2 diabetes, can increase the risk of PC (16–18). However, it is unclear which aspect of MetS is most strongly associated with PC and whether gender influences the effect of MetS on PC. The effects of MetS as a risk factor on PC were thoroughly reviewed and subjected to a meta-analysis. Furthermore, sub-analyses based on gender were conducted.
Methods and materials
Search strategy
PubMed, Embase, and the Cochrane Library databases were systematically searched for pertinent studies that were published between the creation of the database and November 1, 2022. The following search terms were used: (‘pancreatic carcinoma’ OR ‘pancreatic cancer’ OR ‘pancreatic adenocarcinoma’ OR ‘pancreatic neoplasms’) AND (‘metabolic syndrome’ OR ‘Metabolic X Syndrome’ OR ‘Dysmetabolic Syndrome X’ OR ‘MetS’). Furthermore, the reference lists of qualified articles were visually examined for any additional pertinent studies.
Selection criteria
Based on the inclusion and exclusion criteria listed below, two researchers screened the retrieved publications independently, and discrepancies were settled by consensus. The following inclusion criteria were applied: (1) the publication that was written in English and was a cohort study or a case-control study; (2) data on the relative risk (RR), odds ratio (OR), or hazard ratio (HR) with a 95% confidence interval (95%CI) were available; (3) when multiple publications were produced from the same data, only the most comprehensive paper was selected.
Exclusion criteria for this study were as follows: (1) letters, case reports, reviews, expert opinions, or editorials were excluded; (2) excluded if they lacked critical data; (3) excluded if they failed to mention MetS and diagnostic criteria for PC explicitly; or (4) they were duplicates of other studies. Additionally, case-control studies were excluded from the meta-analysis but included in the systematic review.
Quality assessment
The Newcastle-Ottawa Scale (NOS) for quality evaluation of cohort studies and case-control studies was used to independently evaluate study quality (19). The NOS comprises eight components assigned to three groups based on selection, comparability, and research type exposure (case-control studies) or outcome (cohort studies). For each issue, a number of response alternatives were offered. A star system was employed to provide a semi-quantitative evaluation of the quality of the study. The highest-quality studies yielded a maximum of one star for each item, with the exception of the comparability item, which makes two stars. The NOS stars range between zero to nine. We discussed any disagreements until an agreement was reached. After examination, it was concluded that each study under investigation was of moderate to high quality.
Data extraction
The names of the first authors, the year the study was published, the country where the investigation was done, the duration of follow-up, the total number of patients, and the criteria for the definition of MetS were all retrieved separately by the two researchers for each study that was accessible. Using the most adjusted model, we derived the pooled risk estimates and associated 95%CIs. A discussion was used to settle any disagreements. To assess the effects of MetS components on the risk of developing PC, risk estimates were also gathered for each individual MetS component.
Statistical analyses
Using pertinent risk estimations, the relative risks (RR), hazard ratios (HRs), incident rate ratios, standardized incidence ratios (SIRs), and their 95%CIs were employed to evaluate the relationship between MetS and PC risk. From the multivariable models of the original studies, adjusted risk estimates were generated. Additionally, we assessed how each component of the metabolic syndrome affected the risk of PC on an individual basis. Sensitivity analysis was also carried out to test whether any of the studies had shown a significant impact on the outcome. Using the random-effects model, the outcomes of the retrieved papers were combined. In order to evaluate the statistical heterogeneity across studies, the I2 statistic was used. Low, moderate, and high levels of heterogeneity were estimated to be 25%, 50%, and 75%, respectively. To assess publication bias, the Egger test and funnel plotting were performed. When at least ten original publications were included, a P value < 0.05 showed publication bias. STATA (version 16.0) was used for conducting all analyses, and statistical significance was established at P< 0.05.
Results
Search results
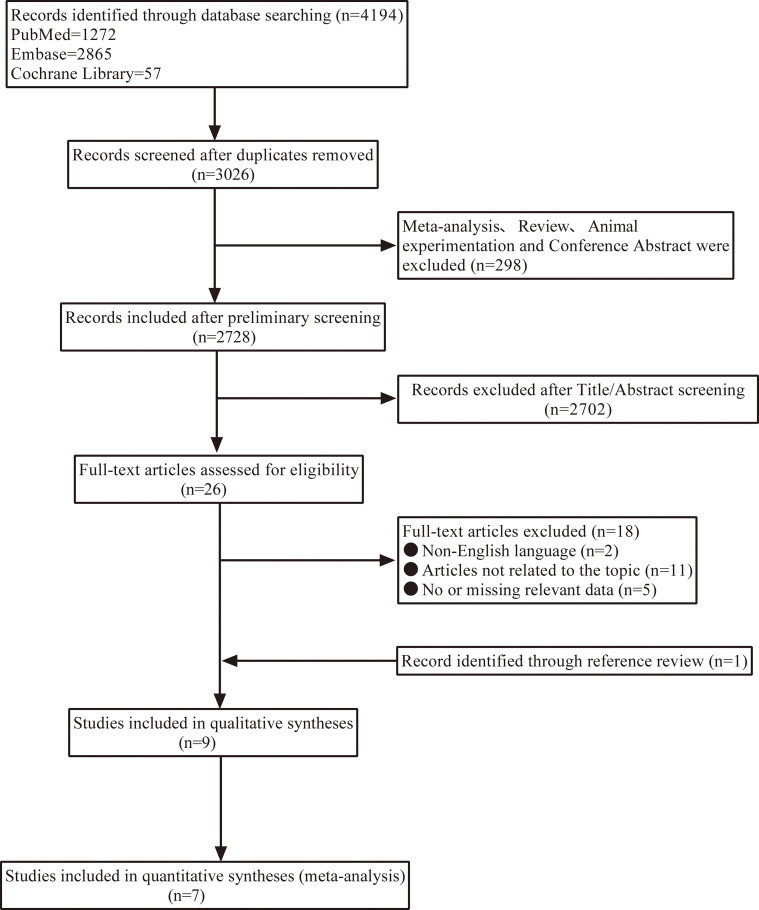
Figure 1 displays a flow chart that illustrates the literature screening process. In total, 4,194 articles were retrieved from databases. Nine publications (12–15, 20–24), comprising two case-control studies (13, 23) and seven cohort studies (12, 14, 15, 20–22, 24), were considered in the systematic review. All the duplicate studies and those studies that failed to meet the inclusion criteria were eliminated. Meta-analysis was performed on all cohort studies ( Figure 1 ).
Figure 1.
Flowchart for screening the literature.
Characteristics of included studies
A complete summary of the fundamental characteristics of each study that was included in this research is provided in Table 1 . The study comprises research published between 2008 and 2022, and their quality scores, on average, were 7.2 stars. The median follow-up period per a study in the included literature ranged from 2.7 (Russo et al.) to 10.2 (Manami Inoue et al.) years. The adjusted analyses showed varied potential confounding factors (risk factors), including a maximum of 10 (21, 24) and a minimum of 5 confounders (15). In addition, only four studies reported an association between high blood glucose, blood pressure, triglyceride levels, and HDL-c levels with PC (12, 14, 22, 24). In comparison, five studies reported an association of obesity with PC (12, 14, 21, 22, 24).
Table 1.
Characteristics of the studies included in the quantitative and qualitative review.
| Author | Year | Country | Study Type | Age (range or mean) |
MetS criteria | Follow-up | Sample size | No. of cases | Quality assessment |
|---|---|---|---|---|---|---|---|---|---|
| Antonio Russo (20) | 2008 | Italy | Cohort | ≥40 | Pharmacological definition | median follow-up 2.7 years | 16,677 | 43 | 6 |
| Manami Inoue (15) | 2009 | Japan | Cohort | M:56.5 ± 8.2 F:55.5 ± 8.1 |
AHA | average follow-up 10.2 years | 27,724 | 65 | 6 |
| Valentina Rosato (13). | 2011 | Italy | Case control | 34-80 | AHA | 17years | 978 | 21 | 6 |
| Bin Xia (12). | 2020 | China | Cohort | MetS (+):58.1 MetS (-):55.8 |
IDF | MetS (+):6.5 years(1.3) MetS (-):6.6 years(1.2) |
475,078 | 565 | 8 |
| Sung Keun Park (14) | 2020 | South Korea | Cohort | MetS (+):60.3 ± 9.1 MetS (-):56.92 ± 8.4 |
IDF | 4years | 222,838 | 381 | 8 |
| HyeSoo Chung (21) | 2021 | South Korea | Cohort | MetS (+):60 ± 9 MetS(-):59.3 ± 8.7 |
IDF | median follow-up 6.1 years | 347,434 | 886 | 7 |
| Joo-Hyun Park (22) | 2022 | South Korea | Cohort | 48.9 | IDF | median follow-up 5.1 years | 8,203,492 | 8010 | 8 |
| Joseph A (24) | 2022 | French | Cohort | MetS (+):58.40 ± 7.61 MetS(-):55.48 ± 8.15 |
NCEP-ATPIII | median follow-up 7.1 years | 366,494 | 478 | 8 |
| Tomàs López-Jiménez (23) | 2022 | Spain | Case control | ≥40 | AHA | 11years | 183,284 | 1996 | 8 |
AHA, American Heart Association;
IDF, International Diabetes Federation;
NCEP-ATP III, National Cholesterol Education Program Adult Treatment Panel III.
Meta-analysis results
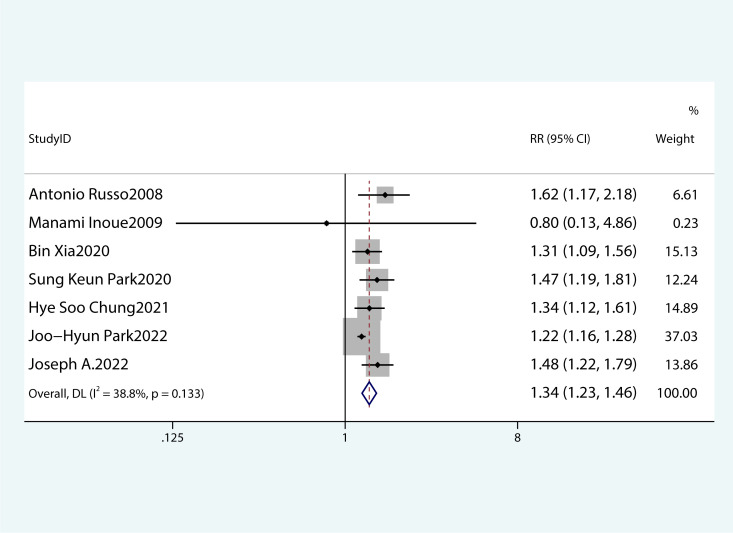
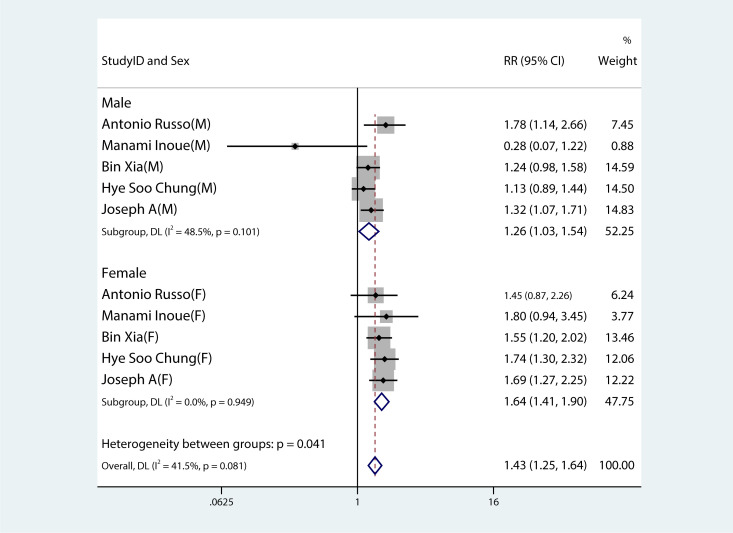
Figures 2 – 8 show forest plots for the PC and MetS meta-analysis. In comparison with non-MetS individuals, patients having MetS had a greater probability of getting PC (RR 1.34, 95%CI 1.23–1.46, P<0.0001, I2 = 38.8%) ( Figure 2 ). For the subgroup analysis of the prevalence of PC in MetS patients, the study population was divided into male and female groups. It was observed that the prevalence of PC was remarkably higher in males and females with MetS than among non-MetS patients. Among MetS patients, females were more likely to develop PC than males (male: RR 1.26, 95%CI 1.03–1.54, P=0.022; females: RR 1.64, 95%CI 1.41–1.90, P< 0.001) ( Figure 3 ).
Figure 2.
Meta-analysis of studies on the correlation of metabolic syndrome with pancreatic cancer;.
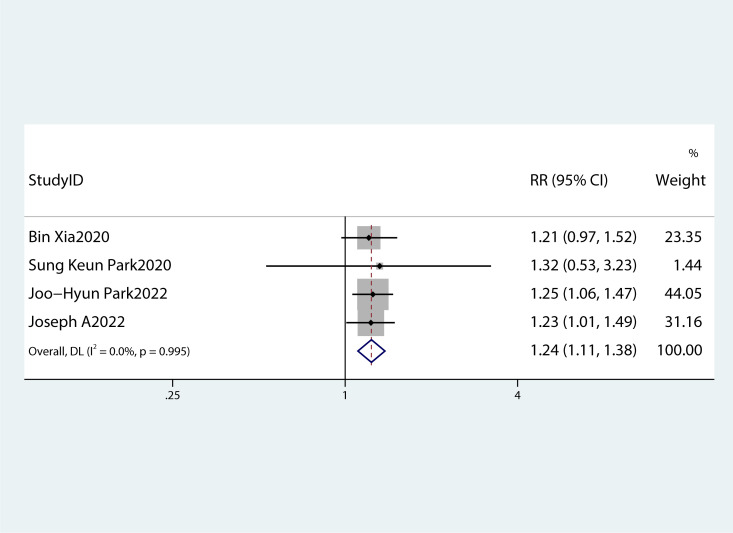
Figure 8.
A forest plot demonstrating the relationship between low HDL-c levels and the risk of pancreatic cancer.
Figure 3.
Forest plot demonstrating the association between the metabolic syndrome and the risk of developing pancreatic cancer in both males and females.
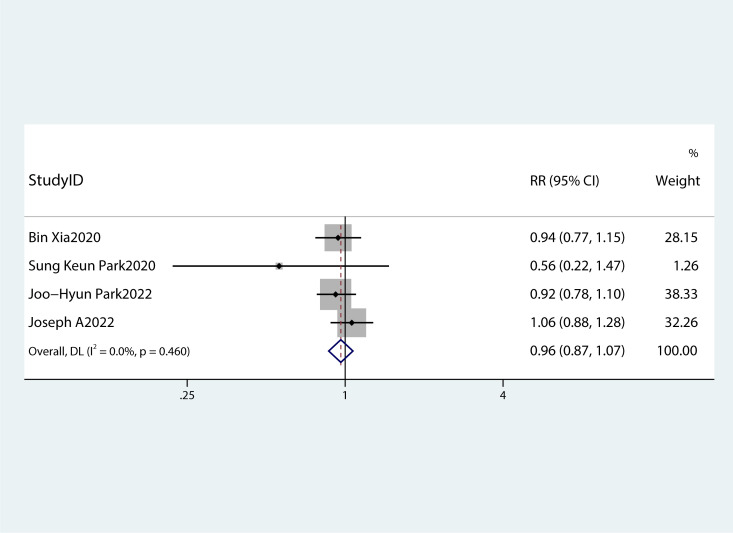
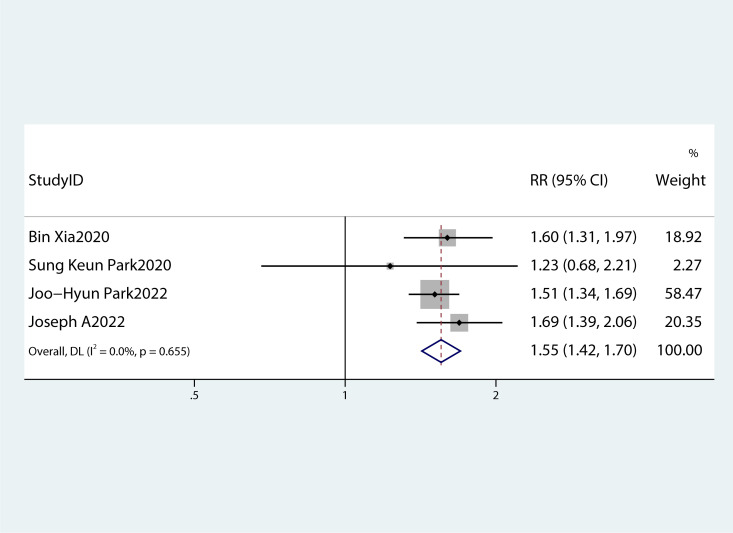
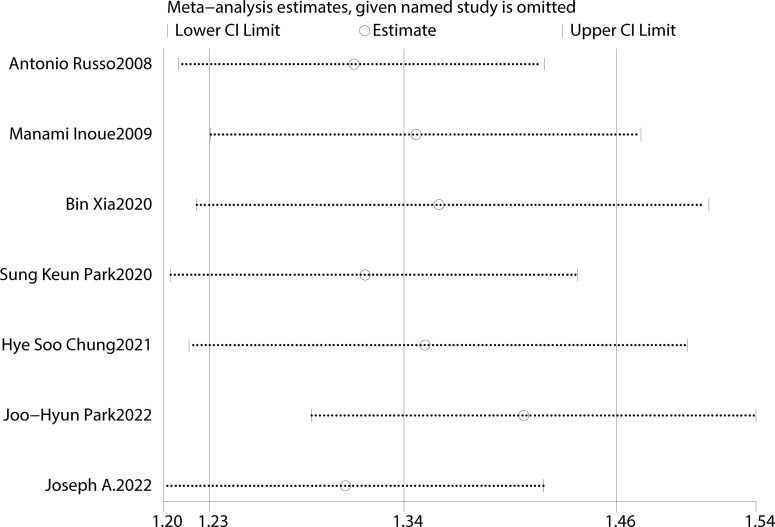
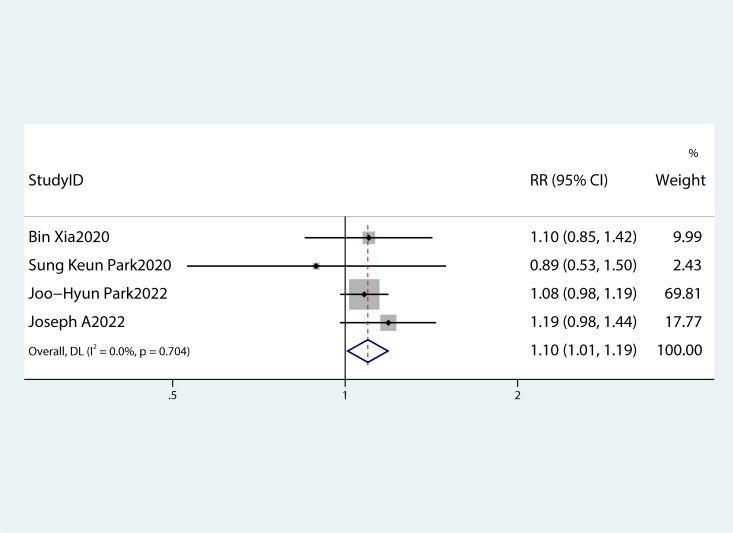
Table 2 summarizes the diagnostic criteria for every single component of MetS present in each study, and Table 3 lists the numerous types of diagnostic criteria for MetS. These findings demonstrated that the risk of PC was not correlated with obesity or hypertriglyceridemia (obesity: RR 1.13, 95%CI 0.96–1.32, P=0.151; hypertriglyceridemia: RR 0.96, 95%CI 0.87–1.07,P=0.486) ( Figures 4 , 5 ).While hyperglycemia, hypertension and low HDL-c increased the risk of PC (hyperglycemia: RR 1.55,95%CI 1.42–1.70, P< 0.001; hypertension: RR 1.10, 95%CI 1.01–1.19, P=0.027; low HDL-c: RR 1.24, 95%CI 1.11–1.38, P< 0.001) ( Figures 6 – 8 ). The results of the sensitivity analysis showed that the link between MetS and the risk of PC was unaffected noticeably due to the lack of any studies ( Figure 9 ).
Table 2.
Diagnostic criteria for any single component of metabolic syndrome in each study.
| Author | Year | hypertension | hyperglycemia | obesity | hypertriglyceridemia | Low HDL-c |
|---|---|---|---|---|---|---|
| Antonio Russo (20) | 2008 | Use of drugs for hypertension | Use of drugs for diabetes | - | - | Use of drugs for hypercholesterolemia |
| Manami Inoue (15) | 2009 | BP≥130/85 mmHg and/or use of antihypertensive agents |
glucose ≥ 5.55 mmol/l (100 mg/dl) fasting or ≥ 7.77 mmol/l (140 mg/dl) non- fasting |
BMI ≥ 25 kg/m2 | high serum triglycerides ≥ 1.69 mmol/l (150 mg/dl) | low HDL-c< 1.03 mmol/l (40 mg/dl) for men and <1.29 mmol/l (50 mg/dl) for women |
| Bin Xia (12) | 2020 | systolic ≥ 130 mmHg or diastolic ≥ 85 mmHg or treatment of previously diagnosed hypertension | FPG ≥ 100 mg/dLor previously diagnosed type 2 diabetes | BMI > 30 kg/m2 | TGlevels ≥ 0.7 mmol/L (150 mg/dL) or currently on medications for hypertriglyceridaemia |
HDL-c< 0.9 mmol/L (40 mg/dL) for men and < 1.29 mmol/L (50 mg/dL) in women or specific treatment for previously detected reduced HDL -c. |
| Sung Keun Park (14) | 2020 | BP ≥130/85 mm Hg | FPG ≥ 100 mg/dL | WC≥90 cm in men and ≥85 cm in women |
TG levels ≥150 mg/dL | HDL-c< 40 mg/dL for men and < 50 mg/dL for women |
| Hye Soo Chung (21) | 2021 | BP ≥130/85 mmHg or the use of antihypertensive agents |
FPG≥5.6 mmol/L (100 mg/dL) or useofan antidiabetic drug | BMI is ≥25 kg/m2 | serum triglyceride levels ≥1.7 mmol/L (≥150 mg/dL) or the current use of lipid-lowering agents |
HDL-c <1.0 mmol/L (40 mg/dL) in men or<1.3 mmol/L (50 mg/dL) in women or the current use of lipid-lowering agents |
| Joo-Hyun Park (22) | 2022 | systolic ≥130 or diastolic ≥80 mmHg or the use of antihypertensive agents |
FPG≥100 mg/dL or the use of an antidiabetic drug | WC≥90 cm in men and ≥85 cm in women |
TG levels ≥150 mg/dL or the use of a relevant drug | HDL-C <40 mg/dL for men and <50 mg/dL for womenor the use of a relevant drug |
| JosephA (24) | 2022 | systolic ≥ 130 mmHg and diastolic ≥ 85 mmHg, or previously diagnosed high BP, or regular use of BP-lowering medication. |
HbA1c≥ 5.7%, regardless of diabetes status. | WC≥102 cm in men or ≥88 cmin women | triglycerides were considered elevated if measured at ≥1.7 mmol/L | reduced HDL was defined as ≤1.03 mmol/L in men and ≤1.29 mmol/L in women, or regular use of cholesterol-lowering medication |
BP, blood pressure; FPG, fasting plasma glucose; BMI, body mass index; WC, waist circumference; TG, plasma triglyceride; HDL-c, high-density lipoprotein cholesterol.-, It means that the diagnostic criteria for this component of metabolic syndrome are notprovided in the article.
Table 3.
Different Criteria for MetS Diagnosis.
| MetS Diagnosis Criterion | Details |
|---|---|
| Pharmacological definition | Patients who are also taking medicine for high cholesterol, high blood pressure, and diabetes |
| NCEP-ATP III | (1) WC≥102 cm in men and ≥88 cmin women; (2) TG≥1.7 mmol/L; (3) HDL-c ≤ 1.03mmol/L in men and ≤1.29 mmol/L in women (4) BP≥130/85 mmHg; (5) FPG≥6.1 mmol/L; ≥3 above components can be diagnosed as MetS. |
| IDF | (1) central obesity(WC ≥ 90 cm and ≥ 80 cm in Asians, with other values for other ethnicities; or BMI > 30 kg/m2); (2) TGlevels ≥ 0.7 mmol/L (150 mg/dL); (3) HDL-c< 0.9 mmol/L (40 mg/dL) for men and < 1.29 mmol/L (50 mg/dL) in women or specific treatment for previously detected reduced HDL -c; (4) systolic ≥ 130 mmHg or diastolic ≥ 85 mmHg or treatment of previously diagnosed hypertension; (5) FPG ≥ 100 mg/dLor previously diagnosed type 2 diabetes; central obesity plus any two of the above four factors can be diagnosed as MetS. |
| AHA | (1) FPG ≥ 100 mg/dL or receiving drug therapy for hyperglycemia; (2) BP ≥ 130/85 mmHg or receiving drug therapy for hypertension; (3) TG ≥ 150 mg/dL or receiving drug therapy for hypertriglyceridemia; (4) HDL-c < 40 mg/dL in men or < 50 mg/dL in women or receiving drug therapy for reduced HDL-C; (5) WC≥90 cm in men or ≥80 cmin women; ≥3 above components can be diagnosed as MetS. |
NCEP-ATP III, National Cholesterol Education Program Adult TreatmentPanel III;
IDF, International Diabetes Federation;
AHA, American Heart Association;
BP, blood pressure;
FPG, fasting plasma glucose;
BMI, body mass index;
WC, waist circumference;
TG, plasma triglyceride;
HDL-c, high-density lipoprotein cholesterol.
Figure 4.
A forest plot demonstrating the relationship between obesity and the risk of pancreatic cancer.
Figure 5.
A forest plot demonstrating the relationship between hypertriglyceridemia and the risk of pancreatic cancer.
Figure 6.
A forest plot demonstrating the relationship between hyperglycemia and the risk of pancreatic cancer.
Figure 9.
Sensitivity analyses for pancreatic cancer;.
Figure 7.
A forest plot demonstrating the relationship between hypertension and the risk of pancreatic cancer.
Discussion
Among the components of MetS, dyslipidemia, hypertension, diabetes, and obesity-related biological processes are closely related to one another and increase the risk of developing numerous diseases. The strongest risk factor for PC is diabetes, which is one of the various components that constitute MetS (13). According to UK Biobank data, the PC risk was increased in people with MetS (HR = 1.31, 95%CI 1.09–1.56), hyperglycemia (HR = 1.60, 95%CI 1.31–1.97), and abdominal obesity (HR = 1.24, 95%CI 1.02–1.50). However, these two last MetS components (central obesity and hyperglycemia) seem to exhibit an independent connection in increasing the risk of PC (12).
The present study indicated a correlation between MetS and the risk of PC. The hypothesis of this study was supported by the two case-control studies that were part of the systematic review. Low degrees of study heterogeneity were observed, however. Through subgroup analyses, the cause of heterogeneity was identified, and we came to the conclusion that among MetS patients, the risk of developing PC was higher in women than in men. This observation was consistent with the findings of one of the previous studies (25).
Moreover, a summary of each MetS component’s impact on PC risk was produced. According to the majority of studies (26, 27), PC risk is correlated with hypertension, hyperglycemia, low HDL-c levels, and particularly with hyperglycemia.
There has been extensive research on the pathogenesis of PC in diabetes mellitus or hyperglycemia. PC cells multiplied and invaded as a result of p38 MAPK elicited by high glucose levels. Additionally, P38 MAPK was also activated as a result of cellular stress and inflammatory conditions, which could control metastasis, apoptosis, and cell proliferation. PC cell proliferation and development occurred as a result of heightened paracrine effects of inflammatory cytokines (such as IL-6) and VEGF, which were mediated by P38 MAPK. Moreover, elevated hyperglycemia via RET (a proto-oncogene that encodes a receptor tyrosine kinase for members of the glial cell line-derived neurotrophic factor family of extracellular signaling molecules) can boost PC cell invasion and proliferation (18). Meta-analyses had also shown that dietary cholesterol might be linked to a higher risk of PC (28), which was confirmed by the results of this study. Surprisingly, obesity and hypertriglyceridemia were not associated with PC in the meta-analysis. Previous studies also revealed that there is an increased risk of developing cancer due to obesity (17, 29–31), contradictory to the outcomes of this study. Evidence suggested that the development and progression of PC were caused by an increase in various hormones in obese people, including insulin, adipokines, and resistin (18). Resistin is an adipocyte-secreting hormone involved in insulin resistance and inflammation. It has the ability to affect the progression of the PC. In patients with pancreatic ductal adenocarcinoma, it was considered a negative independent prognostic factor for relapse-free survival (32). Therefore, we speculate that the possible reason for this is insulin resistance and/or low HDL-c levels in most obese individuals, which can increase the cancer risk. Moreover, as per the outcomes of a meta-analysis performed in 2012, the body mass index and central obesity are linked to an average RR of 1.10 for a five-unit rise in the occurrence of PC (33). This correlation applies to African Americans (34) but not to residents of Lithuania (35) or Singapore’s Chinese nonsmoking population (36). Asians comprised the majority of the ethnicities examined in the studies used in the meta-analyses conducted in this research. The European Australasian (RR: 1.18, 95%CI 1.09–1.27) and North American (RR: 1.07, 95%CI 1.03–1.11) populations, however, showed favorable relationships between MetS and PC (37). These outcomes can be explained based on different study methodologies and variations, for example, socioeconomic, genotypic, and environmental aspects of these diverse groups.
Elevated triglyceride levels and reduced HDL-c are the components of MetS. Previous studies on dyslipidemia and the risk of PC produced controversial results (36, 38, 39). In the present study, no evidence of increased risk of developing PC due to high triglyceride levels was obtained.
MetS is reversible. In patients with MetS, and the lifestyle-modification intervention was successful. It resulted in easing the condition and decreasing the severity of associated abnormalities (triglycerides, waist size, systolic and diastolic blood pressure, and fasting blood glucose) (39). Previous results also suggested that MetS could be a risk factor for PC that is modifiable (22). The connection between MetS and the risk of PC may be explained by various molecular pathways. First, insulin resistance is a significant contributor to the pathophysiology of MetS. Elevated insulin levels, as well as modulation of insulin-like growth factors-1 and -2, may contribute to PC by boosting cell proliferation and angiogenesis while inhibiting cell death (40–42). Moreover, visceral adipose tissue has a high metabolic rate and secretes a variety of cytokines that promote inflammation (41, 42). Chroniclow-grade inflammation, including these cytokines, may increase the risk of PC by increased production of reactive oxygen species and cell cycle rates, thus attenuating tumor suppressor activity (42, 43). Finally, MetS have been linked to the altered composition of gut microbiota, decreased microbial diversity, and decreased gene richness, all of which are crucial for carcinogenesis and tumorigenesis (43, 44). Therefore, it can be concluded that preventing or recovering from MetS might reduce the risk of developing PC.
However, this meta-analysis has some limitations. First, like any other meta-analysis, residual confounding from the original studies cannot be eliminated. After correcting for the majority of significant confounding factors, residual or unknown confounders may persist. Because each trial was adjusted for a unique set of variables, meta-analyses may have been heterogeneous. Second, the comprehensiveness of this study was limited by the relatively small number of pertinent publications, which precluded analyses for other relevant characteristics, including age and ethnicity. Third, the metabolic components were not directly assessed using the same technique, which may result in high heterogeneity between studies. However, the sensitivity analysis and subgroup analysis showed the robustness of our outcomes.
In conclusion, our meta-analysis revealed that MetS showed a remarkable correlation with a high risk of developing PC in both genders, with a higher risk in females as compared to males. Low HDL-c levels or hyperglycemia may be primarily responsible for the higher risk of PC in individuals with MetS. However, obesity and hypertriglyceridemia do not increase the risk of PC.
Data availability statement
The original contributions presented in the study are included in the article/ Supplementary Materials . Further inquiries can be directed to the corresponding author.
Author contributions
All authors contributed to the study’s conception and design. LZ was in charge of material preparation, data collection, and analysis. SL wrote the first draft of the paper. JFL contributed to the writing, revision, and review of the manuscript. GT reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1116582/full#supplementary-material
References
- 1. Cai J, Chen H, Lu M, Zhang Y, Lu B, You L, et al. Advances in the epidemiology of pancreatic cancer: Trends, risk factors, screening, and prognosis. Cancer letters (2021) 520:1–11. doi: 10.1016/j.canlet.2021.06.027 [DOI] [PubMed] [Google Scholar]
- 2. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA: Cancer J Clin (2016) 66(2):115–32. doi: 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: Cancer J Clin (2019) 69(1):7–34. doi: 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 4. Midha S, Chawla S, Garg PK. Modifiable and non-modifiable risk factors for pancreatic cancer: A review. Cancer letters (2016) 381(1):269–77. doi: 10.1016/j.canlet.2016.07.022 [DOI] [PubMed] [Google Scholar]
- 5. Reaven GM. Banting lecture 1988. role of insulin resistance in human disease. (1997) 13(1):65. doi: 10.1016/0899-9007(97)90878-9 [DOI] [PubMed] [Google Scholar]
- 6. Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res practice (2014) 2014:943162. doi: 10.1155/2014/943162 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7. Fahed G, Aoun L, Bou Zerdan M, Allam S, Bou Zerdan M, Bouferraa Y, et al. Metabolic syndrome: Updates on pathophysiology and management in 2021. Int J Mol Sci (2022) 23(2):786. doi: 10.3390/ijms23020786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arthur RS, Kabat GC, Kim MY, Wild RA, Shadyab AH, Wactawski-Wende J, et al. Metabolic syndrome and risk of endometrial cancer in postmenopausal women: A prospective study. Cancer causes control CCC (2019) 30(4):355–63. doi: 10.1007/s10552-019-01139-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dibaba DT, Ogunsina K, Braithwaite D, Akinyemiju T. Metabolic syndrome and risk of breast cancer mortality by menopause, obesity, and subtype. Breast Cancer Res Treat (2019) 174(1):209–18. doi: 10.1007/s10549-018-5056-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee J, Lee KS, Kim H, Jeong H, Choi MJ, Yoo HW, et al. The relationship between metabolic syndrome and the incidence of colorectal cancer. Environ Health Prev Med (2020) 25(1):6. doi: 10.1186/s12199-020-00845-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mariani M, Sassano M, Boccia S. Metabolic syndrome and gastric cancer risk: A systematic review and meta-analysis. Eur J Cancer Prev (2021) 30(3):239–50. doi: 10.1097/CEJ.0000000000000618 [DOI] [PubMed] [Google Scholar]
- 12. Xia B, He Q, Pan Y, Gao F, Liu A, Tang Y, et al. Metabolic syndrome and risk of pancreatic cancer: A population-based prospective cohort study. Int J cancer (2020) 147(12):3384–93. doi: 10.1002/ijc.33172 [DOI] [PubMed] [Google Scholar]
- 13. Rosato V, Tavani A, Bosetti C, Pelucchi C, Talamini R, Polesel J, et al. Metabolic syndrome and pancreatic cancer risk: A case-control study in Italy and meta-analysis. Metabolism: Clin experimental (2011) 60(10):1372–8. doi: 10.1016/j.metabol.2011.03.005 [DOI] [PubMed] [Google Scholar]
- 14. Park SK, Oh CM, Kim MH, Ha E, Choi YS, Ryoo JH. Metabolic syndrome, metabolic components, and their relation to the risk of pancreatic cancer. Cancer. (2020) 126(9):1979–86. doi: 10.1002/cncr.32737 [DOI] [PubMed] [Google Scholar]
- 15. Inoue M, Noda M, Kurahashi N, Iwasaki M, Sasazuki S, Iso H, et al. Impact of metabolic factors on subsequent cancer risk: Results from a large-scale population-based cohort study in Japan. Eur J Cancer Prev (2009) 18(3):240–7. doi: 10.1097/CEJ.0b013e3283240460 [DOI] [PubMed] [Google Scholar]
- 16. Johansen D, Stocks T, Jonsson H, Lindkvist B, Björge T, Concin H, et al. Metabolic factors and the risk of pancreatic cancer: A prospective analysis of almost 580,000 men and women in the metabolic syndrome and cancer project. Cancer epidemiology Biomarkers Prev (2010) 19(9):2307–17. doi: 10.1158/1055-9965.EPI-10-0234 [DOI] [PubMed] [Google Scholar]
- 17. Bracci PM. Obesity and pancreatic cancer: overview of epidemiologic evidence and biologic mechanisms. Mol carcinogenesis (2012) 51(1):53–63. doi: 10.1002/mc.20778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Quoc Lam B, Shrivastava SK, Shrivastava A, Shankar S, Srivastava RK. The impact of obesity and diabetes mellitus on pancreatic cancer: Molecular mechanisms and clinical perspectives. J Cell Mol Med (2020) 24(14):7706–16. doi: 10.1111/jcmm.15413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 20. Russo A, Autelitano M, Bisanti L. Metabolic syndrome and cancer risk. Eur J Cancer (Oxford Engl 1990) (2008) 44(2):293–7. doi: 10.1016/j.ejca.2007.11.005 [DOI] [PubMed] [Google Scholar]
- 21. Chung HS, Lee JS, Song E, Kim JA, Roh E, Yu JH, et al. Effect of metabolic health and obesity phenotype on the risk of pancreatic cancer: A nationwide population-based cohort study. Cancer epidemiology Biomarkers Prev (2021) 30(3):521–8. doi: 10.1158/1055-9965.EPI-20-1262 [DOI] [PubMed] [Google Scholar]
- 22. Park JH, Han K, Hong JY, Park YS, Hur KY, Kang G, et al. Changes in metabolic syndrome status are associated with altered risk of pancreatic cancer: A nationwide cohort study. Gastroenterology. (2022) 162(2):509–20.e7. doi: 10.1053/j.gastro.2021.09.070 [DOI] [PubMed] [Google Scholar]
- 23. López-Jiménez T, Duarte-Salles T, Plana-Ripoll O, Recalde M, Xavier-Cos F, Puente D. Association between metabolic syndrome and 13 types of cancer in Catalonia: A matched case-control study. PloS One (2022) 17(3):e0264634. doi: 10.1371/journal.pone.0264634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rothwell JA, Jenab M, Karimi M, Truong T, Mahamat-Saleh Y, Ferrari P, et al. Metabolic syndrome and risk of gastrointestinal cancers: An investigation using Large-scale molecular data. Clin Gastroenterol Hepatol (2022) 20(6):e1338–e52. doi: 10.1016/j.cgh.2021.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Esposito K, Chiodini P, Colao A, Lenzi A, Giugliano D. Metabolic syndrome and risk of cancer: A systematic review and meta-analysis. Diabetes Care (2012) 35(11):2402–11. doi: 10.2337/dc12-0336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gullo L, Pezzilli R, Morselli-Labate AM. Diabetes and the risk of pancreatic cancer. New Engl J Med (1994) 331(2):81–4. doi: 10.1056/NEJM199407143310203 [DOI] [PubMed] [Google Scholar]
- 27. Jee SH, Ohrr H, Sull JW, Yun JE, Ji M, Samet JM. Fasting serum glucose level and cancer risk in Korean men and women. Jama. (2005) 293(2):194–202. doi: 10.1001/jama.293.2.194 [DOI] [PubMed] [Google Scholar]
- 28. Wang J, Wang WJ, Zhai L, Zhang DF. Association of cholesterol with risk of pancreatic cancer: A meta-analysis. World J gastroenterology (2015) 21(12):3711–9. doi: 10.3748/wjg.v21.i12.3711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet (London England) (2008) 371(9612):569–78. doi: 10.1016/S0140-6736(08)60269-X [DOI] [PubMed] [Google Scholar]
- 30. Becker AE, Hernandez YG, Frucht H, Lucas AL. Pancreatic ductal adenocarcinoma: Risk factors, screening, and early detection. World J gastroenterology (2014) 20(32):11182–98. doi: 10.3748/wjg.v20.i32.11182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lu L, Mullins CS, Schafmayer C, Zeißig S, Linnebacher M. A global assessment of recent trends in gastrointestinal cancer and lifestyle-associated risk factors. Cancer Commun (London England) (2021) 41(11):1137–51. doi: 10.1002/cac2.12220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jiang CY, Wang W, Yin YL, Yuan ZR, Wang LB. Expression of the adipocytokine resistin and its association with the clinicopathological features and prognosis of pancreatic ductal adenocarcinoma. Oncol letters (2012) 4(5):960–4. doi: 10.3892/ol.2012.865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aune D, Greenwood DC, Chan DS, Vieira R, Vieira AR, Navarro Rosenblatt DA, et al. Body mass index, abdominal fatness and pancreatic cancer risk: A systematic review and non-linear dose-response meta-analysis of prospective studies. Ann Oncol (2012) 23(4):843–52. doi: 10.1093/annonc/mdr398 [DOI] [PubMed] [Google Scholar]
- 34. Bethea TN, Kitahara CM, Sonderman J, Patel AV, Harvey C, Knutsen SF, et al. A pooled analysis of body mass index and pancreatic cancer mortality in african americans. Cancer epidemiology Biomarkers Prev (2014) 23(10):2119–25. doi: 10.1158/1055-9965.EPI-14-0422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kuzmickiene I, Everatt R, Virviciute D, Tamosiunas A, Radisauskas R, Reklaitiene R, et al. Smoking and other risk factors for pancreatic cancer: A cohort study in men in Lithuania. Cancer Epidemiol (2013) 37(2):133–9. doi: 10.1016/j.canep.2012.10.001 [DOI] [PubMed] [Google Scholar]
- 36. Untawale S, Odegaard AO, Koh WP, Jin AZ, Yuan JM, Anderson KE. Body mass index and risk of pancreatic cancer in a Chinese population. PloS One (2014) 9(1):e85149. doi: 10.1371/journal.pone.0085149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang J, Yang DL, Chen ZZ, Gou BF. Associations of body mass index with cancer incidence among populations, genders, and menopausal status: A systematic review and meta-analysis. Cancer Epidemiol (2016) 42:1–8. doi: 10.1016/j.canep.2016.02.010 [DOI] [PubMed] [Google Scholar]
- 38. Shinoda S, Nakamura N, Roach B, Bernlohr DA, Ikramuddin S, Yamamoto M. Obesity and pancreatic cancer: Recent progress in epidemiology, mechanisms and bariatric surgery. Biomedicines. (2022) 10(6):1284. doi: 10.3390/biomedicines10061284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yamaoka K, Tango T. Effects of lifestyle modification on metabolic syndrome: A systematic review and meta-analysis. BMC Med (2012) 10:138. doi: 10.1186/1741-7015-10-138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yunusova NV, Spirina LV, Frolova AE, Afanas'ev SG, Kolegova ES, Kondakova IV. Association of IGFBP-6 expression with metabolic syndrome and adiponectin and IGF-IR receptor levels in colorectal cancer. Asian Pacific J Cancer Prev APJCP (2016) 17(8):3963–9. [PubMed] [Google Scholar]
- 41. Kwon H, Pessin JE. Adipokines mediate inflammation and insulin resistance. Front endocrinology (2013) 4:71. doi: 10.3389/fendo.2013.00071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine network (2006) 17(1):4–12. [PubMed] [Google Scholar]
- 43. Zambirinis CP, Pushalkar S, Saxena D, Miller G. Pancreatic cancer, inflammation, and microbiome. Cancer J (Sudbury Mass) (2014) 20(3):195–202. doi: 10.1097/PPO.0000000000000045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schulz MD, Atay C, Heringer J, Romrig FK, Schwitalla S, Aydin B, et al. High-fat-diet-mediated dysbiosis promotes intestinal carcinogenesis independently of obesity. Nature. (2014) 514(7523):508–12. doi: 10.1038/nature13398 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Materials . Further inquiries can be directed to the corresponding author.