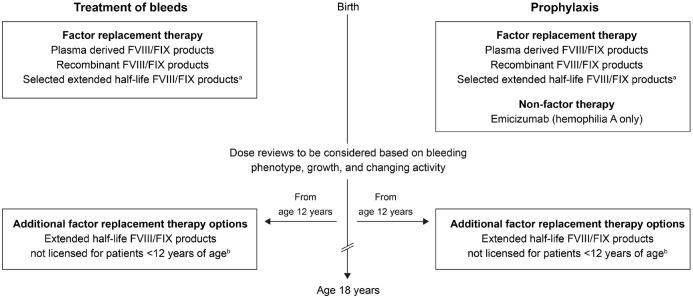
Figure 2.
Treatment options for previously untreated patients (PUPs) with hemophilia A or B who will require prophylaxis. Studies of extended half-life products conducted in PUPs and other pediatric patients are included in Supplemental Table 1.
Product availability will vary between countries. Licensing differs between the European Union and the United States. FVIII, factor VIII; FIX, factor IX; rFVIIIFc, recombinant factor VIII Fc fusion protein; rFIXFc, recombinant factor IX Fc fusion protein; rIX-FP, recombinant factor IX albumin fusion protein.
arFVIIIFc,36,37 rFIXFc,38,39 and rIX-FP40,41 are approved for all age groups in the European Union and the United States; in the United States, rurioctocog alfa pegol, 42 turoctocog alfa pegol, 43 and nonacog beta pegol 44 are also approved for children.
bIn the European Union, use of rurioctocog alfa pegol, 45 turoctocog alfa pegol, 46 damoctocog alfa pegol, 47 and nonacog beta pegol 48 is licensed only in patients ⩾12 years of age; in the United States, damoctocog alfa pegol 49 is licensed only in patients ⩾12 years of age; in both the European Union and the United States, damoctocog alfa pegol47,49 is not licensed for PUPs.