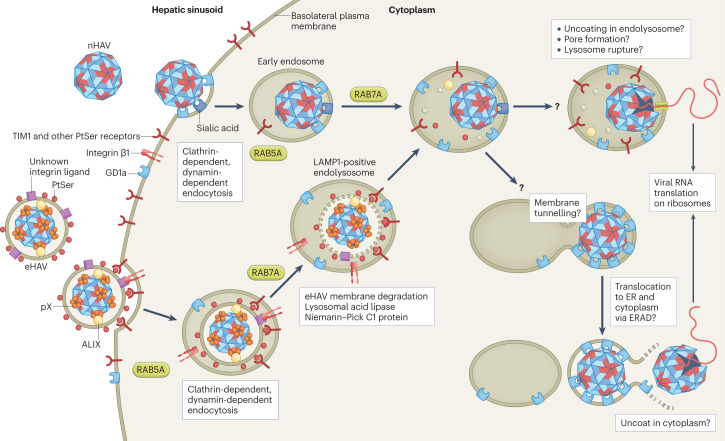
Fig. 3. Cellular entry of naked and quasi-enveloped hepatitis A virus.
Both virion types undergo clathrin-dependent endocytosis driven by interactions between distinct ligands, including phosphatidylserine (PtSer) on the quasi-enveloped hepatitis A virus (eHAV) surface and cellular PtSer receptors such as T cell immunoglobulin mucin receptor 1 (TIM1)68. Endocytosis of naked HAV (nHAV) is inhibited by either sialidase or trypsin treatment of the cell, suggesting that gangliosides and proteins (possibly sialylated) are involved67. Integrin β1, presumably in association with different α-integrins, is required for endocytosis of both virion types. Both virion types traffic through early (RAB5A-positive) and late (RAB7A-positive) endosomal compartments. Subsequent steps that involve uncoating and genome release into the cytoplasm require endolysosomal gangliosides, preferably GD1a (ref. 67). These late steps are delayed for eHAV, which must traffic first to lysosomal-associated membrane protein 1 (LAMP1)-positive endolysosomes, where the quasi-envelope is degraded by lysosomal enzymes and cholesterol transporters, such as lysosomal acid lipase and Niemann–Pick C1 protein. Like nHAV entry, eHAV entry is dependent upon endosomal gangliosides. Subsequent steps in entry are not well understood, but progressive binding of the now-naked capsid to membrane-bound gangliosides may result in tunnelling of the capsid into the membrane. The trigger for capsid disassembly is not known, nor is it known whether uncoating occurs in the endolysosomal lumen or following transport of the capsid across the endolysosomal membrane to the cytoplasm. Entry of naked hepatitis E virus is not dependent upon RAB5A or RAB7A, but entry of quasi-enveloped hepatitis E virus is similar to eHAV entry and also involves trafficking to endolysosomes for degradation of the quasi-envelope60. Receptors have not been identified for the hepatitis E virus capsid. Steps in entry that are not understood are indicated by a question mark. ER, endoplasmic reticulum; ERAD, endoplasmic reticulum-associated degradation pathway.