FIGURE 3.
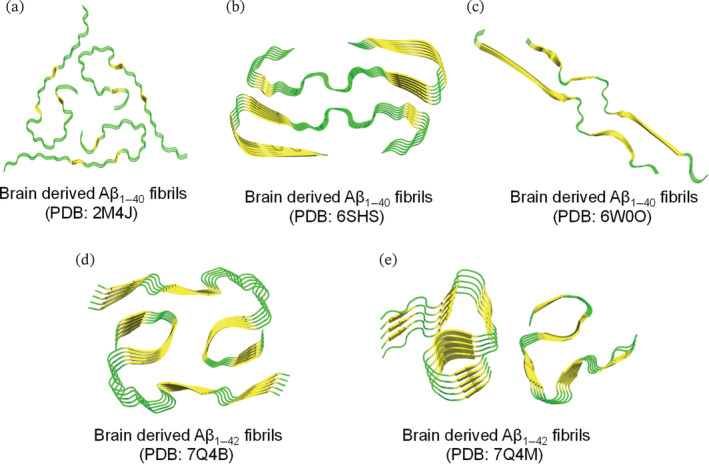
Resolved ssNMR/Cryo‐EM structures of natural brain‐derived Aβ1–42/Aβ1–40 fibrils extracted from deceased AD cases. Top view representation was used to demonstrate the fibril symmetry (i.e., conformation and number of molecules per fibril layer). (a–e) Fibrillar models of brain‐derived fibrils are generated on PyMol using the protein data bank entries and are colored based on secondary structure (yellow for β‐sheets and green for loops). (a) Threefold morphology of brain derived Aβ1–40 fibrils, PDB: 2M4J, from occipital, parietal and temporal cortical AD brain tissues. Structural calculations were performed to accurately determine the fibril structure of Aβ1–40 (PDB: 2M4J) that is fully consistent with the experimental data. (b) Twofold morphology of brain‐derived Aβ1–40 fibrils, PDB: 6SHS, from the meninges of severe AD and CAA (cerebral amyloid angiopathy). (c) Twofold morphology of brain‐derived Aβ1–40 fibrils, PDB: 6W0O, from cortical tissues of AD patients with slightly left‐handed twist as revealed by cryo‐EM. (d and e) Twofold morphologies of brain‐derived Aβ1–42 fibrils, extracted from cortical brain tissues of sporadic (type I in d) and familial (type II in e) AD cases.
