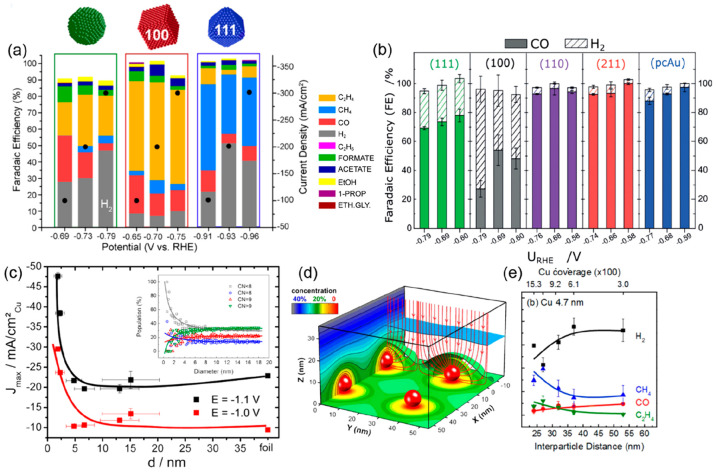
Figure 6.
(a) Relation between the Faradaic efficiencies and potentials for different Cu morphologies (sphere, cube, and octahedra). Reproduced with permission from ref (74). Copyright 2020 American Chemical Society. (b) Relation between the Faradaic efficiencies and potentials with the exposure of different Au facets. Reproduced with permission from ref (75). Copyright 2019 Wiley. (c) Particle size effect during catalytic CO2 electroreduction. The Faradaic current densities at −1.1 and −1.0 V vs. RHE are plotted against the size of the Cu NP catalysts, and the inset shows the population (relative ratio) of surface atoms with a specific coordination number (CN) as a function of the particle diameter. Reproduced with permission from ref (76). Copyright 2014 American Chemical Society. (d) Simulation results of the CO2 concentration distribution based on diffusion equations. The red arrows show the reactant flux toward the NPs. The color scale shows the concentration of CO2 at a given distance from the NPs as a percentage of its value in the bulk of the electrolyte. A diffusion layer thickness of 100 nm was assumed. Reproduced with permission from ref (77). Copyright 2016 American Chemical Society. (e) Faradaic selectivity during the electroreduction of CO2 at −1.1 V vs. RHE with a Cu interparticle distance of 4.7 nm. Reproduced with permission from ref (79). Copyright 2016 American Chemical Society.