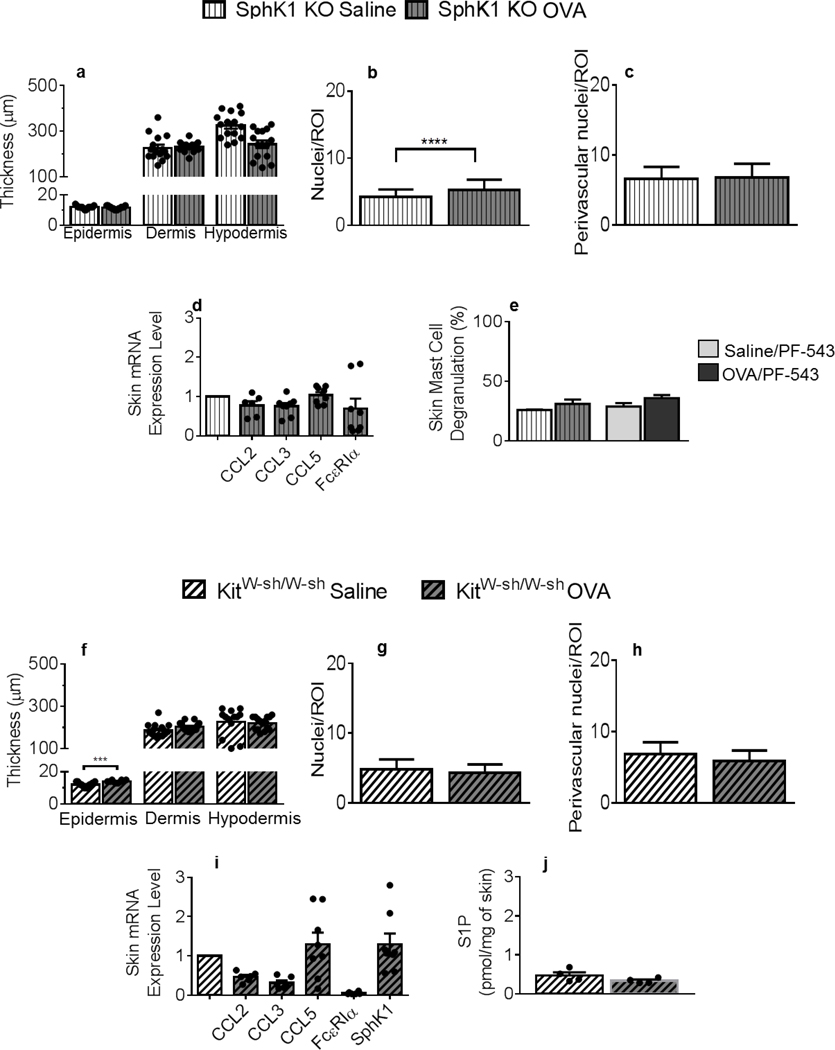
Figure 4. Skin remodeling and chemokine expression are mitigated in mice deficient for SphK1 or mast cells: S1P-mediated skin mast cell degranulation and mast cell-dependent local S1P production.
Thickness of the epidermis, dermis and hypodermis layers in SphK1 KO mice (vertical line patterns) measured in H&E-stained skin tissues. For epidermis thickness, n = 15 (saline, white bars), 15 (OVA, grey bars), for dermis, n = 15 and 13 and for hypodermis, n = 15 and 15 individual measurements collected from 3 animals/treatment group/skin layer. (b) Nuclei numbers per ROI within the hypodermis of saline-treated, compared to OVA-treated SphK1 KO mice. (c) Nuclei numbers per perivascular ROIs after saline or OVA treatment (d) CCL2, CCL3, CCL5 and FcεRIα mRNA expression in OVA-treated (n = 3–4) compared to saline-treated (n = 4) SphK1 KO skins, normalized to GAPDH, with duplicate determinations. (e) Percent mast cell degranulation measured in MB-stained saline- or OVA-treated SphK1-KO or PF-543-treated mouse skin samples. (f) Thickness of the epidermis, dermis and hypodermis layers of KitW-sh/W-sh mice (side-patterned bars), measured in H&E-stained tissues (n = 13–15 saline (white), 10–15 OVA (grey) individual measurements collected from 3 animals/treatment group/skin layer. (g) Nuclei numbers per ROI within the hypodermis of saline-treated compared to OVA-treated KitW-sh/W-sh mice. (h) Nuclei numbers around perivascular ROIs. (i) CCL2, CCL3, CCL5, FcεRIα and SphK1 mRNA expression from OVA-treated normalized to saline-treated KitW-sh/W-sh mouse skin samples, and to GAPDH, with duplicate determinations. (j) S1P content of KitW-sh/W-sh mouse skins after saline or OVA treatment (n = 4 mice/experimental group). All measurements were conducted as described in Figures 1–3. Statistical differences were determined with unpaired 2-tailed Student’s t test with Welch’s correction (a-j). **** p < 0.0001.