Figure 1.
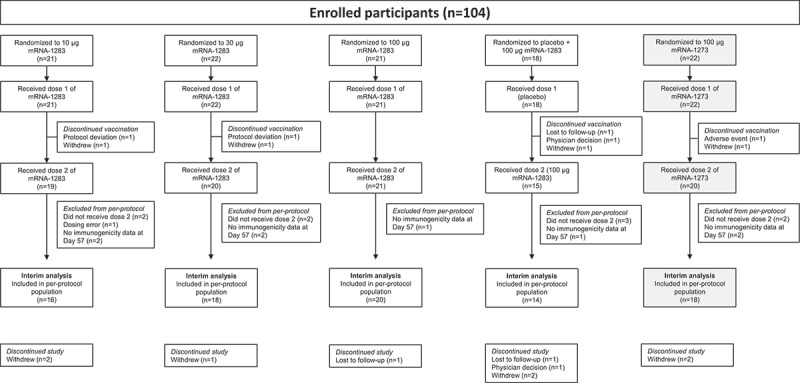
Participant disposition. One participant in the mRNA-1273 100-µg group discontinued vaccination because of an adverse event, which was considered related to study vaccination. All randomly assigned participants who received dose 1 of the study vaccination were included in the safety population. Reasons for exclusion from the per-protocol immunogenicity population are described in the Supplement. Abbreviation: mRNA, messenger RNA.
