Figure 2.
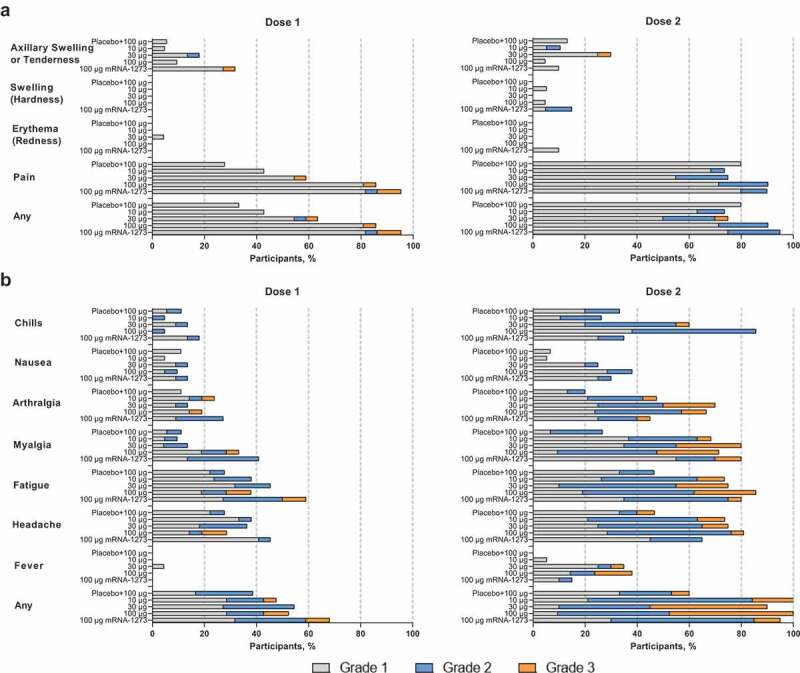
Solicited (a) local and (b) systemic adverse reactions within 7 days of each dose. Percentages are based on participants in the solicited safety population, consisting of all randomly assigned participants receiving ≥1 dose and contributing any solicited adverse reaction data. Number of participants in the dose 1 solicited safety population: mRNA-1283 10 µg, n = 21; mRNA-1283 30 µg, n = 22; mRNA-1283 100 µg, n = 21; placebo + mRNA-1283 100 µg, n = 18; and mRNA-1273 100 µg, n = 22. Number of participants in the dose 2 solicited safety population: mRNA-1283 10 µg, n = 19; mRNA-1283 30 µg, n = 20; mRNA-1283 100 µg, n = 21; placebo + mRNA-1283 100 µg, n = 15; and mRNA-1273 100 µg, n = 20. Abbreviation: mRNA, messenger RNA.
