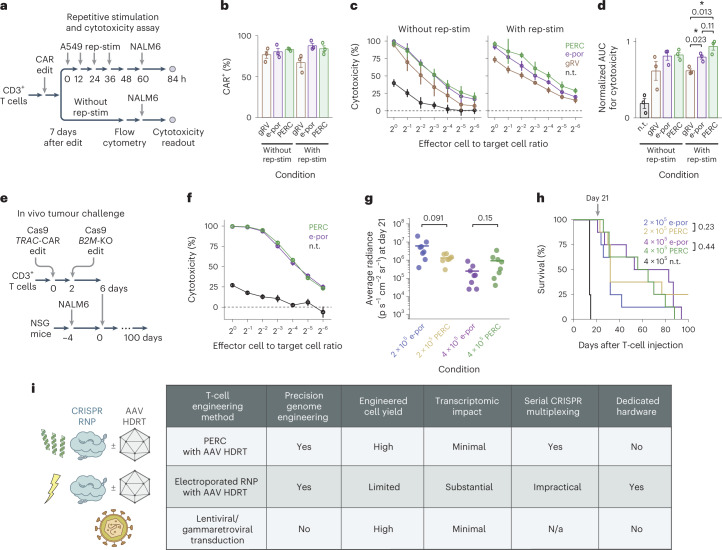
Fig. 5. Functional evaluation of CAR-T cells generated using PERC.
a, Schematic of the repetitive stimulation (rep-stim) and cytotoxicity assay. CD3+ T cells were edited to express a CAR using either gRV, Cas12a RNP electroporation and AAV, or Cas12a RNP PERC and AAV. b, Percentages of CAR+ cells in each condition with or without repetitive stimulation using CD19+ A549 cells. c,d, NALM6 cytotoxicity assay for T cells in each condition (c), and area under the curve analysis (d). n = 3 biological replicates from distinct human donors. Bars represent the mean. Error bars represent s.e.m. (3 biological replicates × 3 technical replicates). P values are from two-tailed Welch’s unpaired t-tests. e, Schematic of the in vivo tumour challenge experiment, using n = 8 mice per group. f, NALM6 cytotoxicity assay. Error bars represent s.e.m. from three technical replicates. g, BLI values on the last measurement day on which all CAR-T-cell-injected mice were alive. P values are from two-tailed Welch’s unpaired t-tests. h, Kaplan–Meier survival analysis. P values are from a log-rank test. P < 0.001 for each comparison of an edited condition versus non-treated (n.t.) cells. i, Table summarizing different delivery methods evaluated.