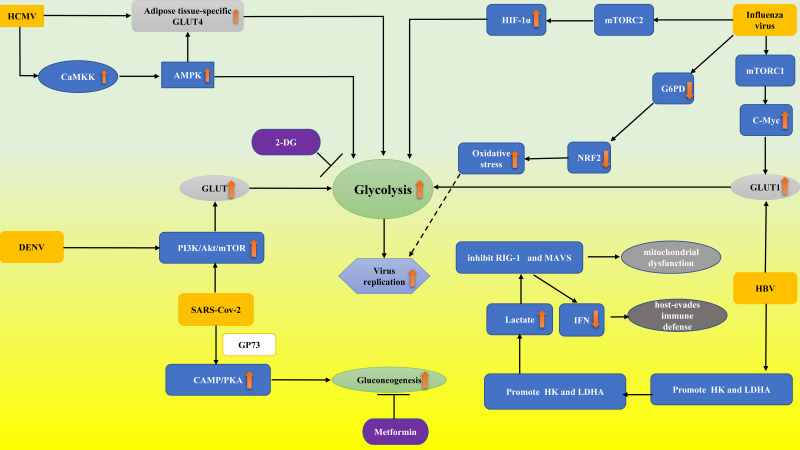
Figure 1.
Abnormal glucose metabolism in virus-associated sepsis. Influenza virus decreased the expression and activity of G6PD and thus downregulated the expression of NRF2, which regulates the gene network of the antioxidant response. Finally, the oxidative stress and virus replication were aggravated. Influenza virus proteins promote glycolysis by stimulating the mTORC1 and mTORC2 pathways by upregulating GLUT1 and HIF-1α expression, respectively. DENV activated the PI3K/Akt/mTOR pathway to upregulate GLUT expression, thus enhancing glycolysis. HCMV resulted in AMPK activation through CaMKK. AMPK activation resulted in glycolytic activation to provide energy and facilitate virus replication. HCMV infection can upregulate GLUT4, which increases glucose uptake. Activated AMPK in turn stimulates glycolysis by GLUT4. HBV achieves glycolytic metabolic recombination by increasing GLUT1 expression. HBV regulates HK activity, and LDHA stimulates lactic acid production, thereby inhibiting RIG-I interaction with MAVS and leading to immune escape by regulating IFN production. SARS-CoV-2 activated the PI3K/Akt/mTOR pathway and led to the release of GP73, which stimulated liver gluconogenesis to enhance fasting glucose through cAMP/PKA. 2-DG and Metformin inhibit viral replication through the mechanisms of suppressing glycolysis and gluconeogenesis, respectively. PI3K/Akt/mTOR pathway, phosphatidylinositide 3-kinases/protein kinase B/mammalian target of rapamycin; cAMP/PKA, cyclic adenosine monophosphate/protein kinase A; CaMKK, calcium/calmodulin dependent kinase; NRF2, nuclear factor erythroid 2-related factor 2; G6PD, glucose-6-phosphate dehydrogenase; 2-DG, 2-deoxy-D-glucose; MAVS, mitochondrial antiviral signaling; HK, hexokinase; RIG-I, retinoic acid-inducing gene I; HIF-1α, hypoxia-inducible factor-1α; mTORC1, rapamycin complex 1; GP73, a type II transmembrane Golgi protein; GLUT4, adipose tissue-specific glucose transporter type 4. The upward orange arrow means upregulation.The downward orange arrow means downregulation.