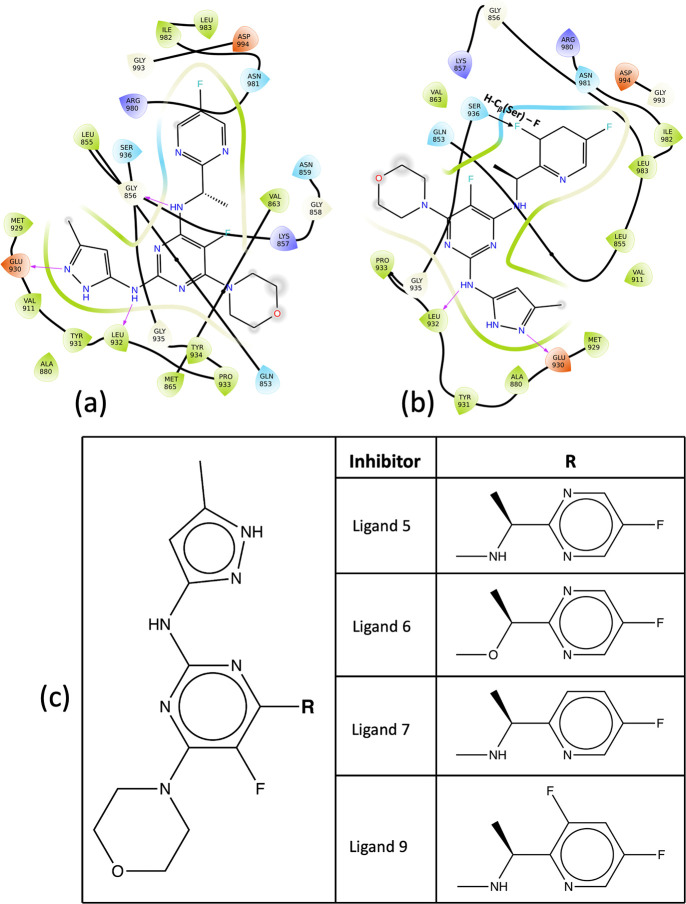
Figure 8.
(a, b) Binding site of inhibitors for JAK2 complex showing important interactions with surrounding residues: (a) JAK2–inhibitor 5 complex and (b) JAK2–inhibitor 9 complex. (c) 2D formulas schemes for the JAK inhibitors indicating the location of modifications. In inhibitor 9, the substituted fluorine atom in the heteroaryl C-ring leads to the electrostatic pull of the hydrogen atom in the nearby serine residue, which contributes to the higher residence time in the kinase domain.